
(a)示意图 (b)制备流程
图1 传感器的示意图及其制备流程
Fig.1 Schematic diagram and its preparation process of the sensor
摘要 “双碳”目标下氢能储运是构建清洁低碳能源体系的重要手段,氨是富氢载体和良好的储氢介质,是大规模、长距离储运氢的有效方式。然而,氨气具有一定的毒性和易燃易爆特性,因此开发高性能氨气传感技术具有重要意义。该文制备了不同掺杂浓度的氮掺杂石墨烯基氨气传感器,并进行了表征与气敏测试,基于第一性原理计算了氮掺杂石墨烯-氨气吸附体系的能量与结构参数,探讨了氮掺杂浓度对传感器性能的影响机理。结果表明:当石墨烯中氮的原子含量百分比小于1.44%时,吡啶氮所占比例较高,其对氨气的吸附能为-0.26 eV,吸附距离为2.637 Å(1 Å=1×10-10 m),转移电荷量为0.04 e(1 e=1.602×10-19 C),吸附性能优于本征石墨烯,可提升传感器性能;超过1.44%时,吡咯氮含量较高,其对氨气的吸附能为-0.08 eV,吸附距离为3.005 Å,转移电荷量为0.02 e,吸附性能比本征石墨烯差,使传感器性能下降。因此,掺杂氮原子含量百分比在1.4%左右时,石墨烯中吡啶氮占主导地位,传感器性能最优。该文可为掺杂浓度对氮掺杂石墨烯基氨气传感器性能的调控提供相应的理论基础。
关键词:石墨烯 氮掺杂 掺杂浓度 第一性原理 氨气传感器
2021年,中国政府提出加快建设包括氢能在内的清洁、低碳、高效、安全的能源体系,由于氨气(NH3)具备17.7%(质量分数)的氢存储[1],且液氨储存要求低,被认为是优质的氢能载体[2]。然而,因其高气化潜热特性[3]与毒性[4],若发生泄漏仍会带来较高的安全风险,故在氨-氢储运过程中对NH3进行准确的监测非常重要。NH3检测的常见方法有色谱法[5]、光谱法[6-7]及传感器法[8]等,其中气体传感器的体积较小且操作简单,是实现NH3在线监测的主要方法[9-14]。
目前,组成NH3传感器的气敏材料主要是金属氧化物半导体[15],但其高工作温度会导致器件漂移、功耗增大[16]。以石墨烯为代表的二维材料,其大比表面积、高载流子迁移率[17]等特性有利于NH3的低温检测。但本征石墨烯对大部分气体存在吸附性能差等问题[18-19],为此,研究人员在材料与气体的吸附原理、材料的组成与结构设计等方面进行了深入研究。Zhang Li等[20]基于第一性原理进行计算,分析了本征石墨烯、硼掺杂石墨烯与氮掺杂石墨烯对NH3的吸附能,发现本征石墨烯与NH3之间的吸附能较弱,硼掺杂与氮掺杂可以增强石墨烯与NH3之间的吸附。Lü Ruitao等[21]以三乙基硼烷/正己烷混合液为前驱体合成的硼掺杂石墨烯可以检测到低浓度的NH3,对于体积分数为1×10-6的NH3,信噪比为50.1,相比于本征石墨烯,其灵敏度提高约105倍。S. Srivastava等[22]采用化学气相沉积(Chemical Vapor Deposition, CVD)法制备了一种氮掺杂石墨烯二氧化氮(NO2)传感器,其对NO2的响应相对于本征石墨烯增大了近3倍。大部分石墨烯气体传感器可逆性差,对此Y. H. Kim等[23]在石墨烯传感器中采用悬挂式微型加热器加快NH3在石墨烯表面的脱附,使传感器的恢复时间缩短。研究表明,氮掺杂可增强石墨烯传感器的NH3气敏性能,但氮掺杂浓度对传感器性能的影响机理仍不明晰。
针对该问题,本文首先制备了不同氮掺杂浓度的石墨烯传感器,对其形貌、结构与化学组分进行了系统表征,分析了氮掺杂浓度对石墨烯结构性能的影响规律;然后,测试了不同掺杂浓度传感器的气敏响应,分析了掺杂浓度对传感器性能的影响;最后,基于第一性原理计算了氮掺杂石墨烯-NH3吸附体系的能量与结构参数,探讨了掺杂浓度对气敏性能的影响机理。
本文制备的NH3传感器为场效应晶体管结构,敏感材料为氮掺杂石墨烯。传感器的示意图及其制备流程如图1所示。采用化学气相沉积法制备氮掺杂石墨烯,甲烷为气态碳源,尿素为固态氮源,铜箔为生长基底。参考本课题组前期的研究成果[24],将生长参数设置为:生长温度为1 030℃、生长时间为5 min、甲烷流量为2 cm3/min、氢气流量为20 cm3/min、氩气流量为300 cm3/min(流量均为标准状态下的数据)。为了后续表征与测试,利用聚甲基丙烯酸甲酯(PMMA)将生长好的石墨烯由铜箔转移至含氧化层的硅片基底。转移后,硅即为场效应管的栅极,源漏电极通过紫外光刻技术、电子束蒸发镀膜等工艺制备,电极材料为金/钛,其中钛用于减小石墨烯与金之间的接触势垒。为使石墨烯与电极形成良好的欧姆接触,将器件在30 cm3/min氢气、300 cm3/min氩气氛围下(流量均为标准状态下的数据)保持350℃退火2 h。最后使用银胶、银线将器件与传感器基座相连,完成传感器的制备。参考先前的研究[25],针对尿素用量对石墨烯结构的影响进行预实验,结果表明使用0、20、30、40 mg的尿素用量梯度时,石墨烯中氮原子含量百分比均匀上升,每相邻两组的含量百分比相差约0.7个百分点,且当尿素用量超过40 mg时,可能导致副反应[26],形成过多的氮氧化物,从而影响石墨烯的结构和性能。因此本文设置尿素为上述四种用量,形成本征石墨烯传感器和三种不同氮掺杂浓度的石墨烯传感器(下文分别简称为PG、NG20、NG30、NG40)。

(a)示意图 (b)制备流程
图1 传感器的示意图及其制备流程
Fig.1 Schematic diagram and its preparation process of the sensor
传感器的结构表征主要针对其敏感材料氮掺杂石墨烯的形貌结构与化学成分进行。利用场发射扫描电镜(Scanning Electron Microscope, SEM)(Gemini SEM 500)观察材料的表面形貌。采用入射波长为514 nm的显微共聚焦拉曼光谱仪(Renishaw inVia)表征材料层数与缺陷程度。对材料厚度使用原子力显微镜(Atomic Force Microscope, AFM)(Bruker Dimension ICON)进行测试。利用X射线光电子能谱(X-ray Photoelectron Spectroscopy, XPS)(Thermo Fisher ESCALB Xi +)研究材料的化学信息。
传感器测量的气敏性能测试系统[27]如图2所示,含配气系统、直流电源(Keithley 2400)、密封测试室、数据采集单元等部分。配气系统由气瓶与多组质量流量控制器(Mass Flow Controller, MFC)(SevenStar)组成,MFC的准确度为满量程的±0.35%,可精准调控背景气(空气)和标准气体(NH3或干扰气体)的流量比,其中空气由纯氮气与纯氧气混合而成,气体流量比为7 3。数据采集单元负责传感器电学指标的实时采集,传感器插入印制电路板后与分压电阻形成串联电路,采集卡测量分压电阻的电压,计算可得传感器的电压、电阻等指标。气体的吸附和脱附即为气体分子与石墨烯之间的电荷转移过程,会引起传感器电学参数变化,分析其变化情况可知传感器的气敏性能。
3。数据采集单元负责传感器电学指标的实时采集,传感器插入印制电路板后与分压电阻形成串联电路,采集卡测量分压电阻的电压,计算可得传感器的电压、电阻等指标。气体的吸附和脱附即为气体分子与石墨烯之间的电荷转移过程,会引起传感器电学参数变化,分析其变化情况可知传感器的气敏性能。
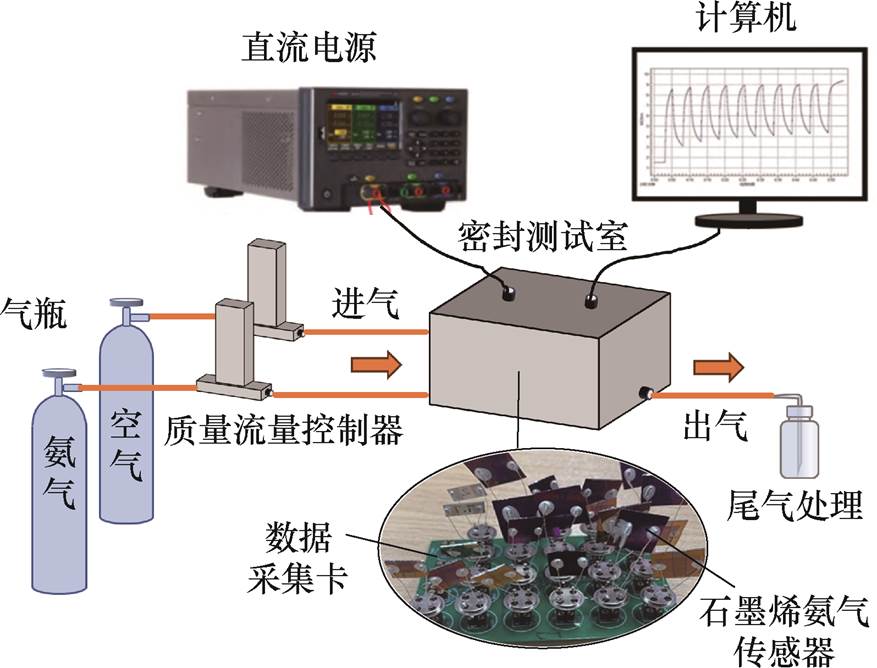
图2 气敏性能测试系统
Fig.2 Test system of gas sensing properties
本文利用基于密度泛函理论的第一性原理计算软件Material Studio建立石墨烯-NH3吸附体系模型,并使用其中的CASTEP模块对吸附体系的吸附能、转移电荷量等参数进行仿真计算。计算采用周期性边界条件;采用广义梯度近似(Generalized Gradient Approximation, GGA)下的交换关联泛函(Perfectly Matched Bases Energies, PBE)描述电子交换关联作用[28],超软赝势描述电子与离子间的物理关联[29],赝势函数采用梯度修正函数,快速傅里叶变换(Fast Fourier Transform, FFT)网格质量设置为标准(Standard),并应用有限基组行为校正;模型结构优化与能量计算时,将布里渊区k-point设置为3×3×1,倒格矢K空间中将电荷密度的平面波截断能设置为240 eV,对晶胞内原子进行完全弛豫,原子间立场收敛精度为5 eV/nm,可承受最大应力设置为0.1 GPa,最大位移大小不超过2×10-4 nm。
氮掺杂石墨烯晶格中的氮原子主要有石墨氮、吡啶氮和吡咯氮三种结构,由于石墨氮在石墨烯晶格中的稳定性低于吡啶氮和吡咯氮,氮原子更倾向于形成吡啶氮和吡咯氮[30-31],本文分别建立NH3与本征石墨烯、吡啶氮掺杂石墨烯、吡咯氮掺杂石墨烯的吸附模型。结构优化后的石墨烯-NH3分子吸附体系如图3所示,图中,d代表石墨烯与氨气分子的吸附距离。由图3可知,吡啶氮掺杂石墨烯含三个吡啶氮原子,吡咯氮掺杂石墨烯含两个吡啶氮原子和一个吡咯氮原子,二者都利用两个吡啶氮原子取代原先的碳原子空位缺陷,可降低石墨烯的形成能,减少计算量。
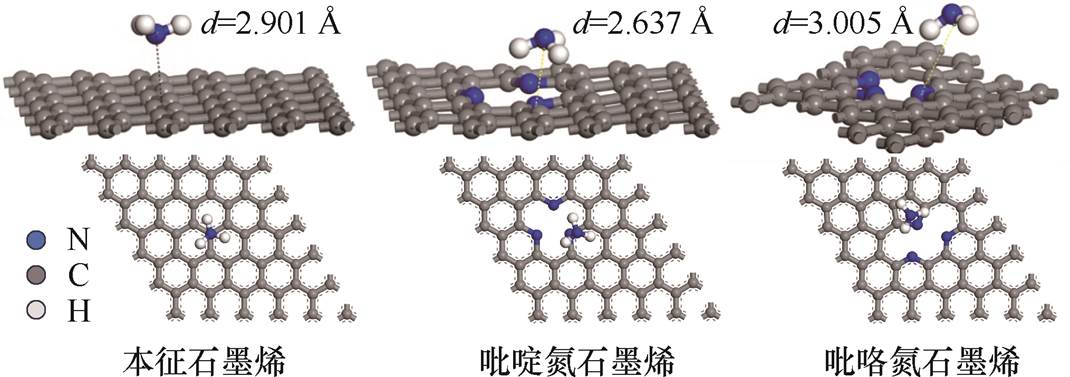
图3 结构优化后的石墨烯-NH3分子吸附体系
Fig.3 The optimized graphene-NH3 molecular adsorption system
NG20、NG30、NG40的SEM表征结果分别如图4a~图4c所示。氮掺杂石墨烯均呈颜色均匀的连续薄膜状态,表面的白色颗粒可能是未完全分解的尿素或受高温而脱落的石英碎屑。图4d和图4e为NG30的AFM表征结果,其厚度为0.894 nm,大于单层石墨烯的标准厚度0.34 nm[32],这是因为制备过程中表面吸附物与杂质的引入导致厚度的测量值比实际值偏大,因此当氮掺杂石墨烯的厚度测试结果在0.5~1 nm间时均可视为单层[33]。图4f为NG30的元素分析结果,可见碳、氮元素的分布密集且均匀,氮原子成功地掺入石墨烯中且材料在基底上均匀分布。
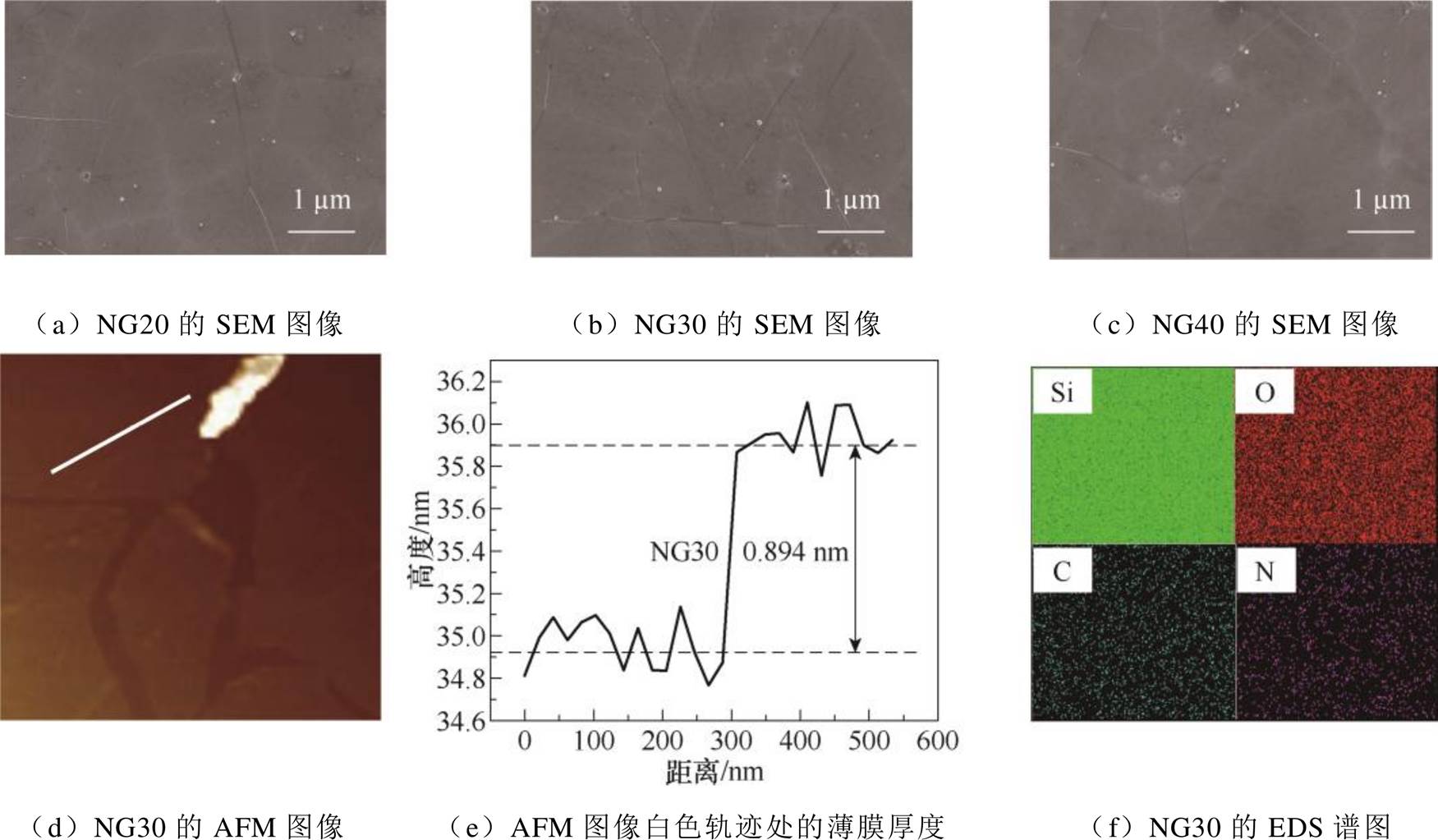
图4 不同尿素用量的氮掺杂石墨烯的表征结果
Fig.4 Characterization results of nitrogen-doped graphene with different urea dosage
三种氮掺杂石墨烯的拉曼光谱如图5所示,氮掺杂石墨烯的特征峰(D峰约位于1 350 cm-1,G峰约位于1 580 cm-1,2D峰约位于2 690 cm-1)随尿素用量的增加发生规律性变化。氮掺杂石墨烯的D峰增强,G峰附近出现伴生的D峰,说明氮掺杂使石墨烯出现由晶格缺陷引起的二阶双共振拉曼散射过程。2D峰变低变宽,2D峰与G峰的峰强比I2D IG减小,这是由费米能级绝对值的上升引起的。G峰表现出红移,且NG40的红移(2.56 cm-1)大于NG20的红移(0.80 cm-1)。发生红移的原因是C—C环尺寸与掺杂石墨烯电子结构的变化[34],这说明氮原子成功地进入石墨烯晶格,且随着尿素用量增加,石墨烯的掺杂程度增大。
IG减小,这是由费米能级绝对值的上升引起的。G峰表现出红移,且NG40的红移(2.56 cm-1)大于NG20的红移(0.80 cm-1)。发生红移的原因是C—C环尺寸与掺杂石墨烯电子结构的变化[34],这说明氮原子成功地进入石墨烯晶格,且随着尿素用量增加,石墨烯的掺杂程度增大。
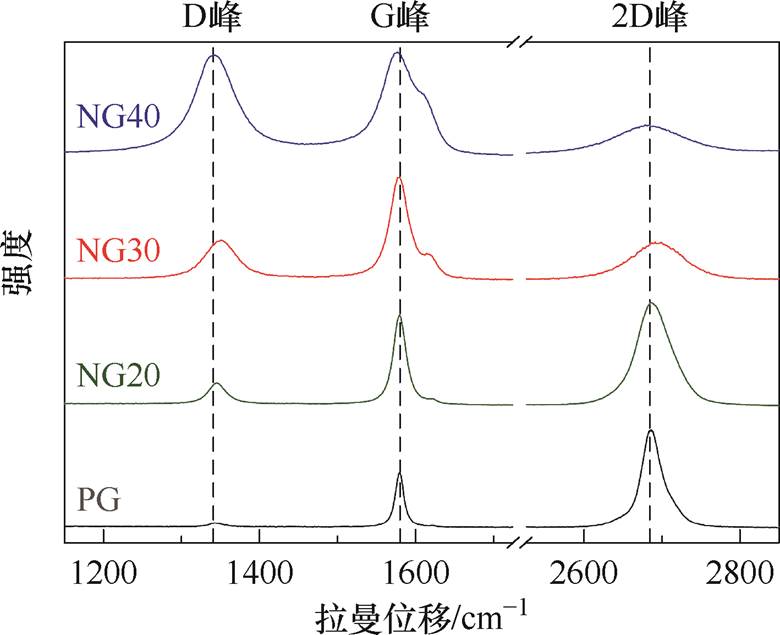
图5 氮掺杂石墨烯的拉曼光谱
Fig.5 Raman spectroscopic characterization of nitrogen-doped graphene
下文利用加热电阻丝设置加热条件(150℃)下工作的NG30传感器为对照组,同时研究氮掺杂浓度与加热对传感器性能的影响。
2.2.1 响应与选择性
传感器对NH3的响应R定义[35]为
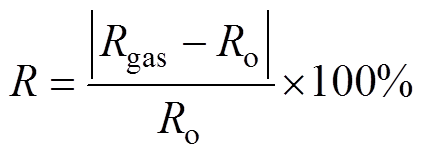 (1)
(1)
式中,Rgas为通入NH3时传感器的电阻值;Ro为通入空气时传感器的电阻值。20℃时传感器对不同浓度NH3的动态响应曲线如图6a所示,其对NH3的响应随石墨烯的氮掺杂浓度的上升先增加后降低,NG30传感器的响应最高,体积分数为100×10-6的NH3中NG30传感器的响应为8.8%,因此后续部分测试以NG30传感器为主。图6b为加热时NG30传感器对NH3的动态响应曲线,与未加热相比,传感器的响应有一定程度的升高,体积分数为100×10-6的NH3中NG30传感器的响应变为11.7%。
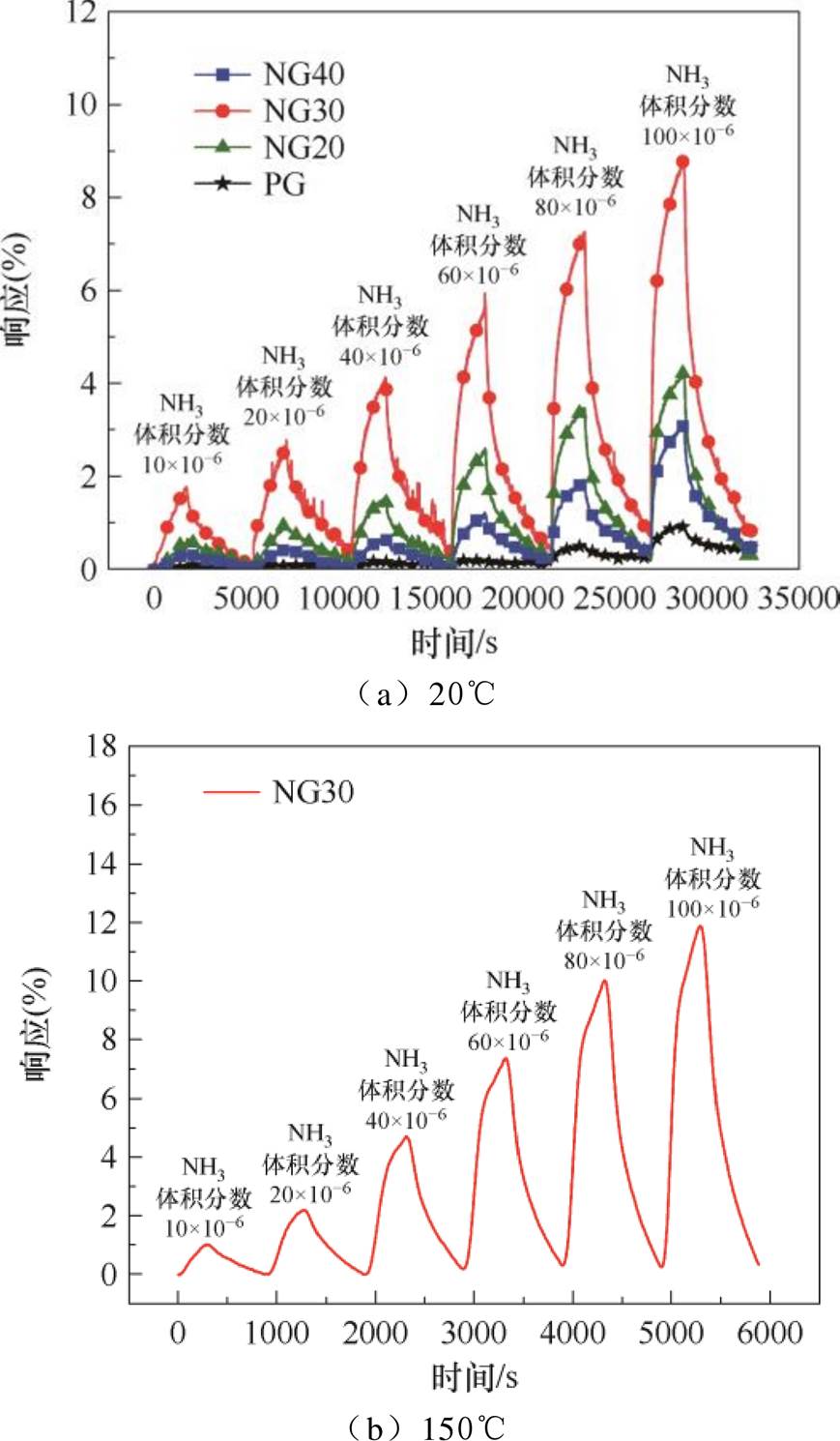
图6 传感器对NH3的动态响应曲线
Fig.6 Dynamic response curves of sensor to NH3
加热时传感器对不同浓度NH3的响应恢复曲线如图7所示。将响应时间tres和恢复时间trec分别定义为达到响应最大值的90%和10%所需的时间,则响应/恢复时间为373/568 s。
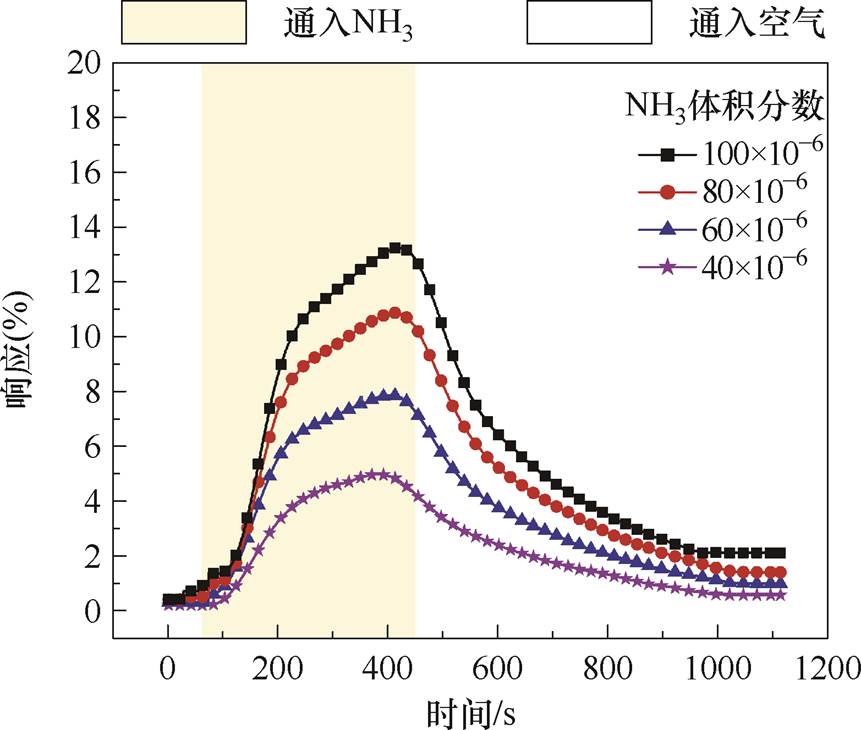
图7 传感器对NH3的响应恢复曲线
Fig.7 Response curves of sensor to NH3
传感器的选择性响应采用选择性系数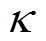 评价[36],计算式为
评价[36],计算式为
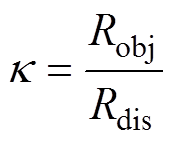 (2)
(2)
式中,Robj为传感器对NH3的响应;Rdis为传感器对干扰气体的响应。依据相关标准,选择体积分数均为50×10-6的NO2、H2、SO2三种气体作为干扰气体[37-40],分别测试传感器在体积分数为50×10-6的NH3及三种干扰气体下的响应。传感器对NH3的选择性测试结果如图8所示。加热前后PG传感器对四种测试气体的响应均较小,对NH3不具有突出的选择性;NG30传感器则显示出对NH3的良好选择性,加热时对NO2、H2与SO2三种气体的选择性系数分别为13、80、8。
需要注意的是,NH3与NO2同为强极性气体,但传感器对二者的响应却存在差异,该现象可能与本文制备的石墨烯样品导电类型(n型或p型)有关。研究表明,石墨烯长时间置于空气中会吸附氧气,氧气夺取石墨烯中的电子成为O-或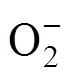 并吸附在表面,在石墨烯中留下大量空穴,使其表现p型半导体特性[41-44]。NH3为还原性气体,倾向于给吸附材料表面提供电子;而NO2为氧化性气体,倾向于从吸附材料表面夺取电子[45]。当p型石墨烯处于还原性气体氛围中时,气体与预吸附的O-或
并吸附在表面,在石墨烯中留下大量空穴,使其表现p型半导体特性[41-44]。NH3为还原性气体,倾向于给吸附材料表面提供电子;而NO2为氧化性气体,倾向于从吸附材料表面夺取电子[45]。当p型石墨烯处于还原性气体氛围中时,气体与预吸附的O-或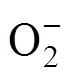 反应释放的自由电子中和了材料中的空穴,导致空穴载流子减少,从而使传感器电阻增加。由于空穴为p型半导体的主要载流子,故传感器对还原性气体的响应较氧化性气体(从材料中夺取电子)更为明显[46],具体的反应过程将在后文展示。本文使用半导体测试仪(Keithley 4200-SCS)在背景气氛围中对四种石墨烯的转移特性进行测试,得到转移特性曲线如图9所示。结果表明,氮掺杂石墨烯的狄拉克点电压Vdirac小于本征石墨烯,即氮掺杂为n型掺杂,但四种石墨烯在背景气氛围中总体均呈现p型半导体特性。结合上述分析,传感器对NH3和NO2响应存在差异可能与石墨烯呈现p型半导体特性有关。
反应释放的自由电子中和了材料中的空穴,导致空穴载流子减少,从而使传感器电阻增加。由于空穴为p型半导体的主要载流子,故传感器对还原性气体的响应较氧化性气体(从材料中夺取电子)更为明显[46],具体的反应过程将在后文展示。本文使用半导体测试仪(Keithley 4200-SCS)在背景气氛围中对四种石墨烯的转移特性进行测试,得到转移特性曲线如图9所示。结果表明,氮掺杂石墨烯的狄拉克点电压Vdirac小于本征石墨烯,即氮掺杂为n型掺杂,但四种石墨烯在背景气氛围中总体均呈现p型半导体特性。结合上述分析,传感器对NH3和NO2响应存在差异可能与石墨烯呈现p型半导体特性有关。
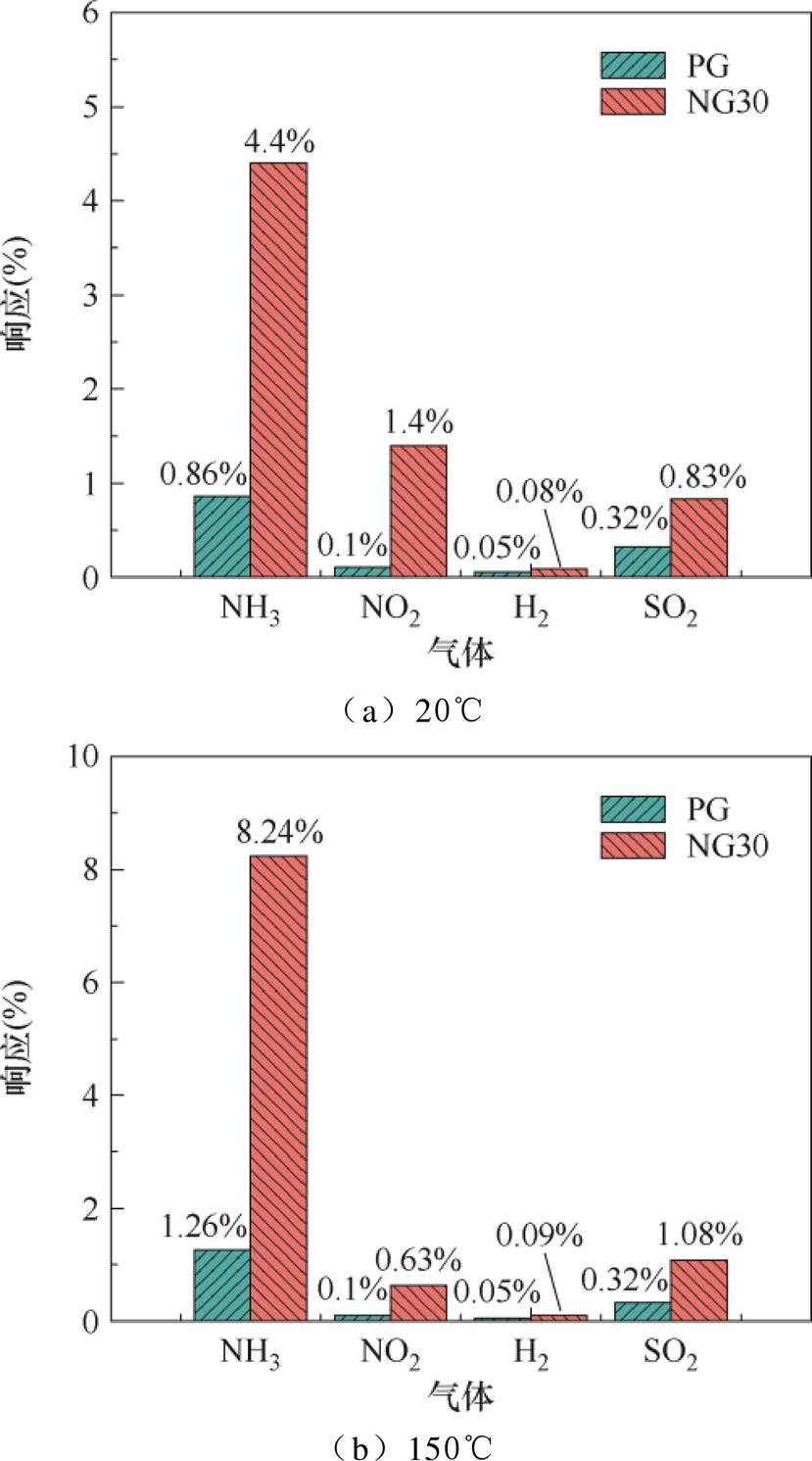
图8 传感器对NH3的选择性测试结果
Fig.8 Selectivity test results of sensor to NH3
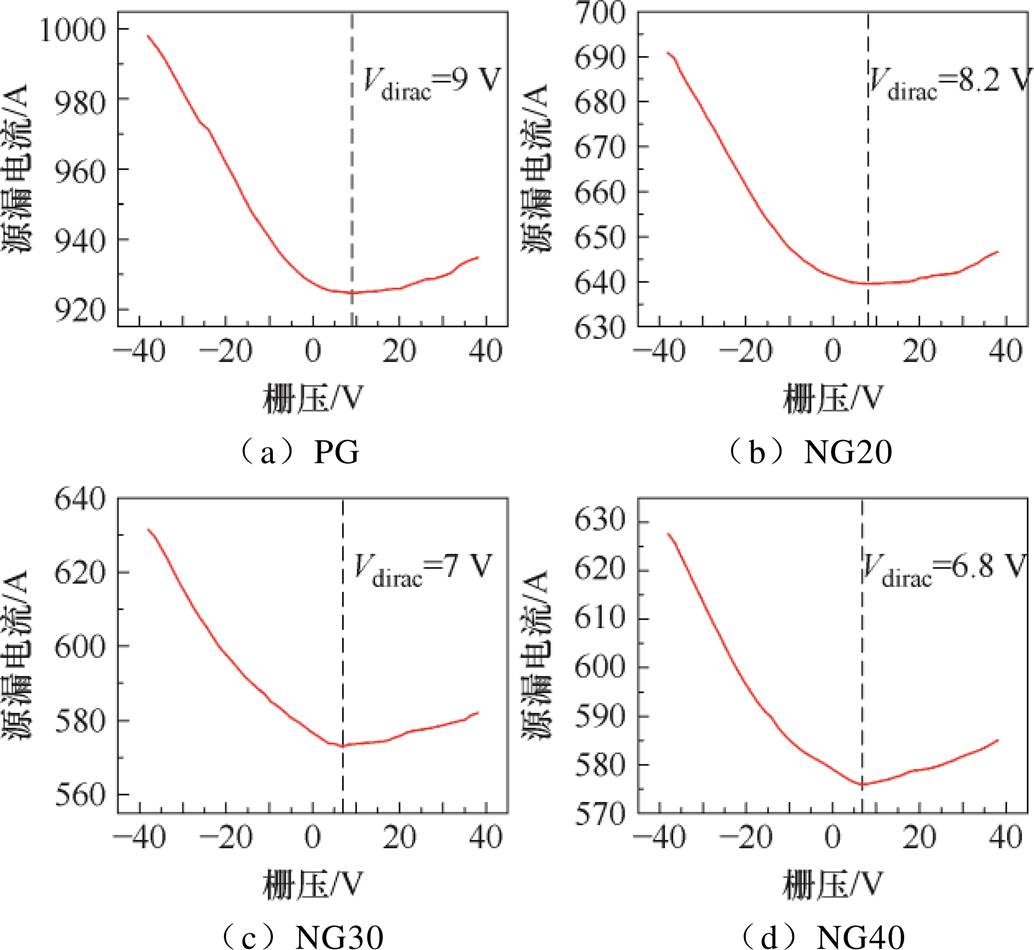
图9 四种石墨烯的转移特性测试结果
Fig.9 Test results of transfer characteristics of four kinds of graphene
2.2.2 灵敏度与最低检测限
四种传感器对不同浓度NH3响应的线性拟合曲线如图10所示。图中,离散点为实际响应,实线为线性拟合曲线。
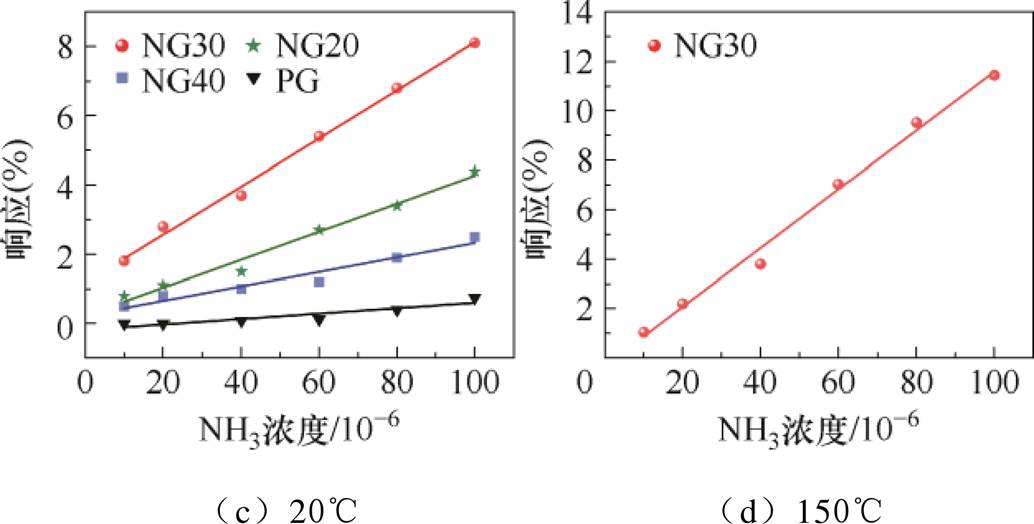
图10 传感器对NH3响应的线性拟合曲线
Fig.10 Linear fitting curve of sensor to NH3
制备的四种传感器对NH3响应曲线的线性拟合结果见表1。由图10和表1可知,PG传感器对NH3的灵敏度最低,为0.007 8%/10-6;吡啶氮含量最高的NG30气体传感器对NH3的灵敏度最高,为0.069 0%/10-6,且线性度最高,线性回归系数(R2)为0.997 74;加热可将NG30传感器的灵敏度进一步提升至0.110 0%/10-6。
表1 传感器响应曲线的线性拟合结果
Tab.1 Linear fitting results of sensor response curves
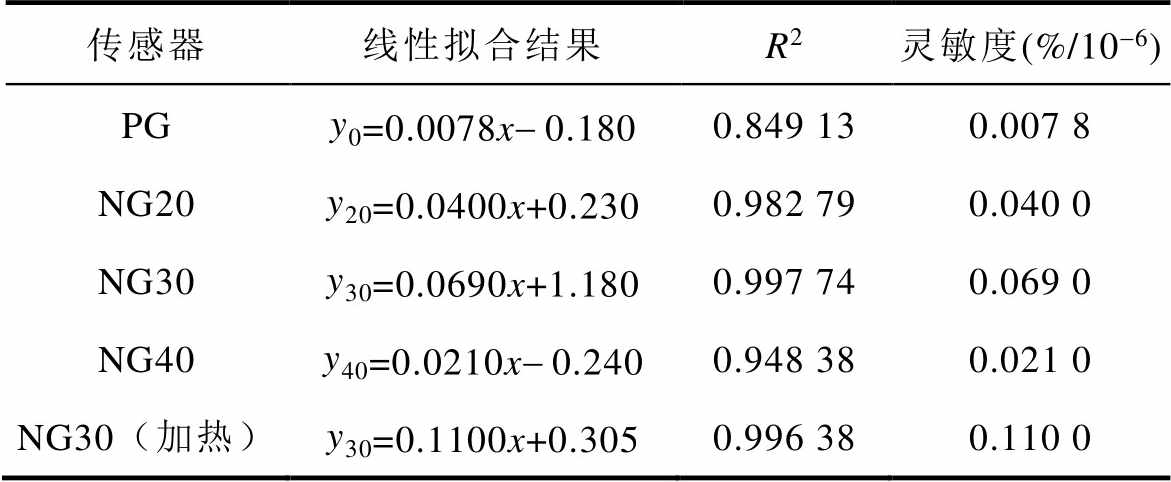
传感器线性拟合结果R2灵敏度(%/10-6) PGy0=0.0078x-0.1800.849 130.007 8 NG20y20=0.0400x+0.2300.982 790.040 0 NG30y30=0.0690x+1.1800.997 740.069 0 NG40y40=0.0210x-0.2400.948 380.021 0 NG30(加热)y30=0.1100x+0.3050.996 380.110 0
最低检测限(Limit of Detection, LOD)是指在规定检测条件下,传感器能准确检测的最小浓度[47],其定义式[48]为
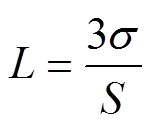 (3)
(3)
式中,L为最低检测限;S为灵敏度;s为基线电阻噪声水平。计算得到NG20、NG30、NG40传感器的噪声水平分别为2.276×10-6、3.220×10-6、4.123× 10-6,NG40传感器的噪声水平最高,这可能是因为掺杂浓度的提升影响了石墨烯内部的电子输运。将噪声水平代入式(3),可得到其LOD分别为171× 10-9、140×10-9、589×10-9,NG30传感器的LOD最低。加热后NG30传感器的噪声水平为2.620×10-5,LOD降低至71×10-9。
NG30传感器对NH3有最高的响应、灵敏度与最低的LOD,掺杂浓度更高的NG40传感器的气敏性能反而下降。因此,下文仅对NG30传感器进行重复性与长期稳定性测试。
2.2.3 重复性与长期稳定性
20℃下NG30传感器对NH3的重复性测试结果如图11a所示。经过4个周期的测试,传感器响应无明显衰减,但无法完全恢复,基线抬升了近0.4%。图11b为加热时的重复性测试结果,各周期响应无明显衰减,且基线漂移幅度比20℃下有所减小,仅抬升了近0.1%。由2.1节中拉曼光谱结果可知,氮掺杂会在石墨烯中引入缺陷和空位,使吸附的NH3分子难以全部与石墨烯分离[49-50],导致传感器存在一定幅度的基线漂移。
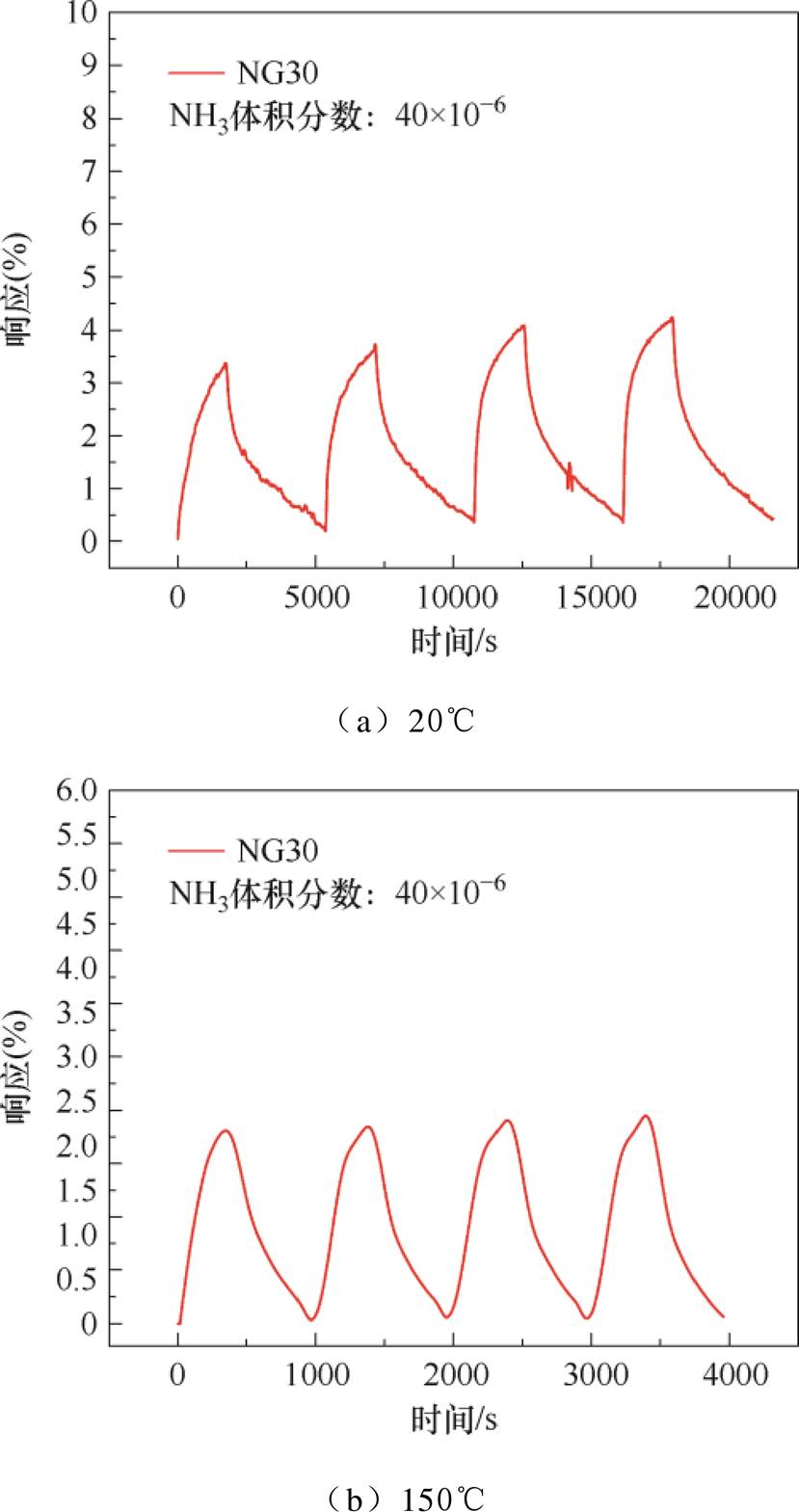
图11 NG30传感器对NH3的重复性测试结果
Fig.11 Repeatability test results of NG30 sensor to NH3
长期稳定性的测试方法为在恒温箱中每2天进行一次NG30传感器对体积分数为100×10-6的NH3的动态响应测试并记录该次测试的最大响应,共测试6次,恒温箱可保持测试期间温度恒定。20℃和150℃下NG30传感器的稳定性测试结果如图12所示。20℃下传感器在10天内对NH3的响应存在大范围波动,以第一次的响应为基准,最大偏差为18.58%,响应的平均下降速率为1.86%/天;加热条件下10天内传感器对NH3响应的最大偏差为7.52%,响应的平均下降速率为0.75%/天,波动范围变小,稳定性增强。结果表明长期测试下,传感器的性能会产生一定程度的下降,这可能是因为氮掺杂在石墨烯引入的缺陷导致NH3分子吸附后难以脱附,后续可通过控制加热温度以及延长稳定性测试时间进一步改善石墨烯基氨气传感器的长期稳 定性。
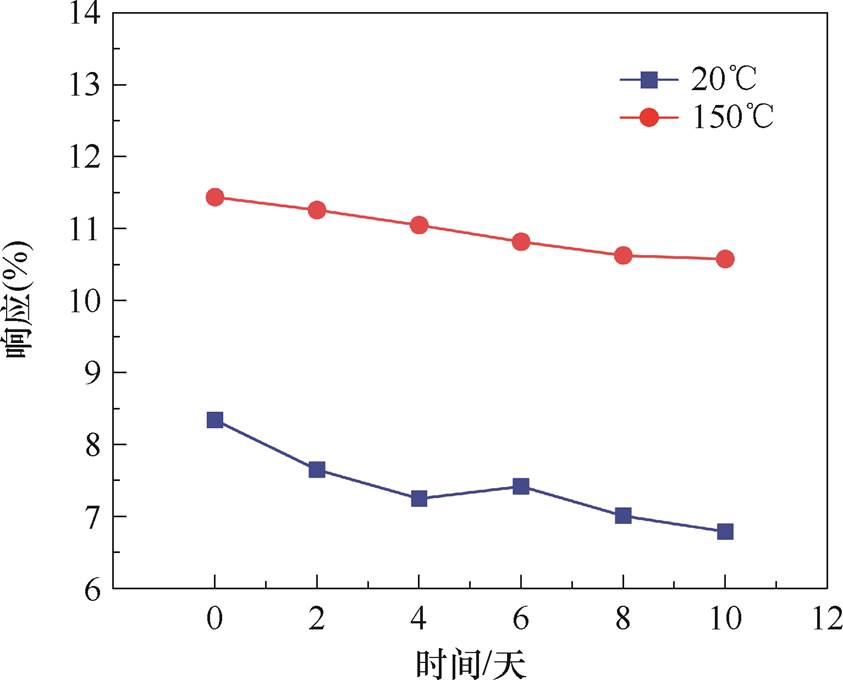
图12 NG30传感器对NH3的长期稳定性测试结果
Fig.12 Stability test results of NG30 sensor to NH3
重复或长期测试会使传感器基线发生漂移且响应减弱,但加热可使石墨烯表面的气体分子接收到更多热能,有助于克服吸附势阱,促进气体分子脱附,使得传感器更快地恢复至基线状态。
将本文制备的NG30传感器与近三年其他文献报道的气体传感器对NH3的气敏性能进行对比,结果见表2。可以看出,本文制备的器件对待测气体有较高的响应,NG30传感器对体积分数为100×10-6的NH3响应达到了11.7%;在最低检测限方面具有独特优势,NG30传感器对NH3的最低检测限达到了71×10-9;而对于响应和恢复时间,本文所制备的气体传感器响应恢复时间较长,但通过加热可极大地缩短该时间。未来通过分析不同加热温度对气体传感器的性能影响,有望进一步提升气体传感器的性能。
表2 氮掺杂石墨烯基NH3传感器性能对比
Tab.2 Performance comparison of nitrogen-doped graphene-based NH3 sensors
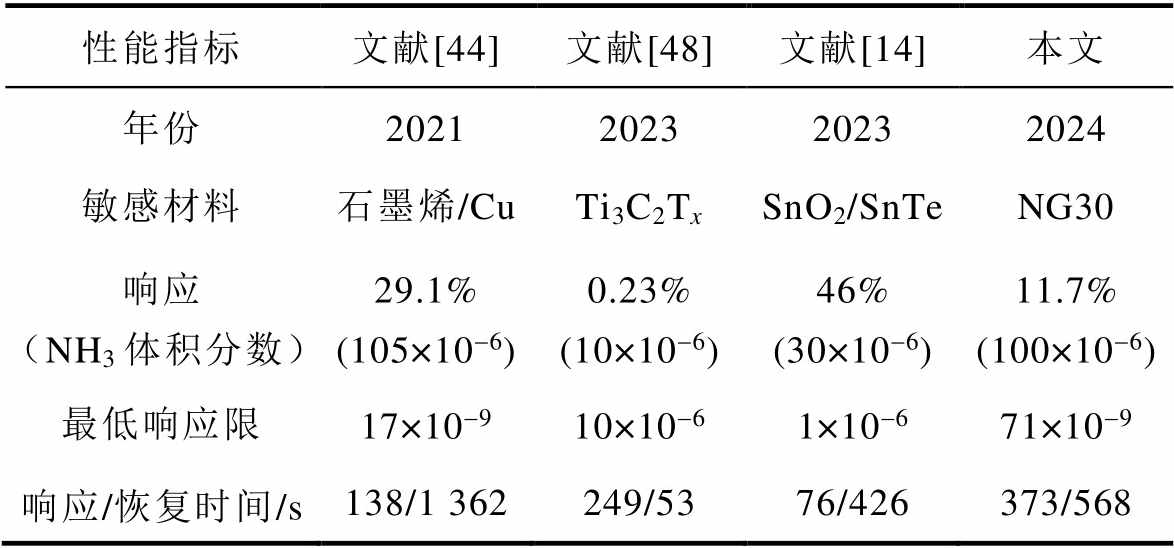
性能指标文献[44]文献[48]文献[14]本文 年份2021202320232024 敏感材料石墨烯/CuTi3C2TxSnO2/SnTeNG30 响应(NH3体积分数)29.1%(105×10-6)0.23%(10×10-6)46%(30×10-6)11.7%(100×10-6) 最低响应限17×10-910×10-61×10-671×10-9 响应/恢复时间/s138/1 362249/5376/426373/568
利用XPS对四种传感器的化学组分进行表征,氮掺杂石墨烯的XPS能谱图如图13所示。图13a为XPS全谱图,其中出现了氮元素特征峰(398 eV),且C 1s轨道峰位于284.7 eV处,说明氮原子在石墨烯晶格中引入了缺陷,使C 1s峰位向高结合能方向移动。图13b为C 1s峰的分峰拟合结果,可得到位于284.7 eV、285.9 eV和287.2 eV三个峰位的分峰分别对应sp2杂化C—C键、sp2杂化C—N键和sp3杂化C—N键[51]。图13c为N 1s峰的分峰拟合结果,可见随着尿素用量增加,氮元素特征峰强度上升,NG20、NG30、NG40的氮原子含量百分比依次为0.76%、1.44%、2.16%,吡啶氮(398.5 eV)的占比分别为87.9%、81.1%、58.1%,吡咯氮(400.1 eV)的占比分别为12.1%、18.9%、41.9%。三种氮掺杂石墨烯中均未检测到石墨烯型氮(401.5 eV)。当尿素用量为20 g或30 g即掺杂浓度较低时,石墨烯中主要为吡啶氮,而当掺杂浓度提高时,吡咯氮的含量增大。说明NG30传感器性能优于NG40传感器的现象可能与各种掺杂氮原子的占比有关。
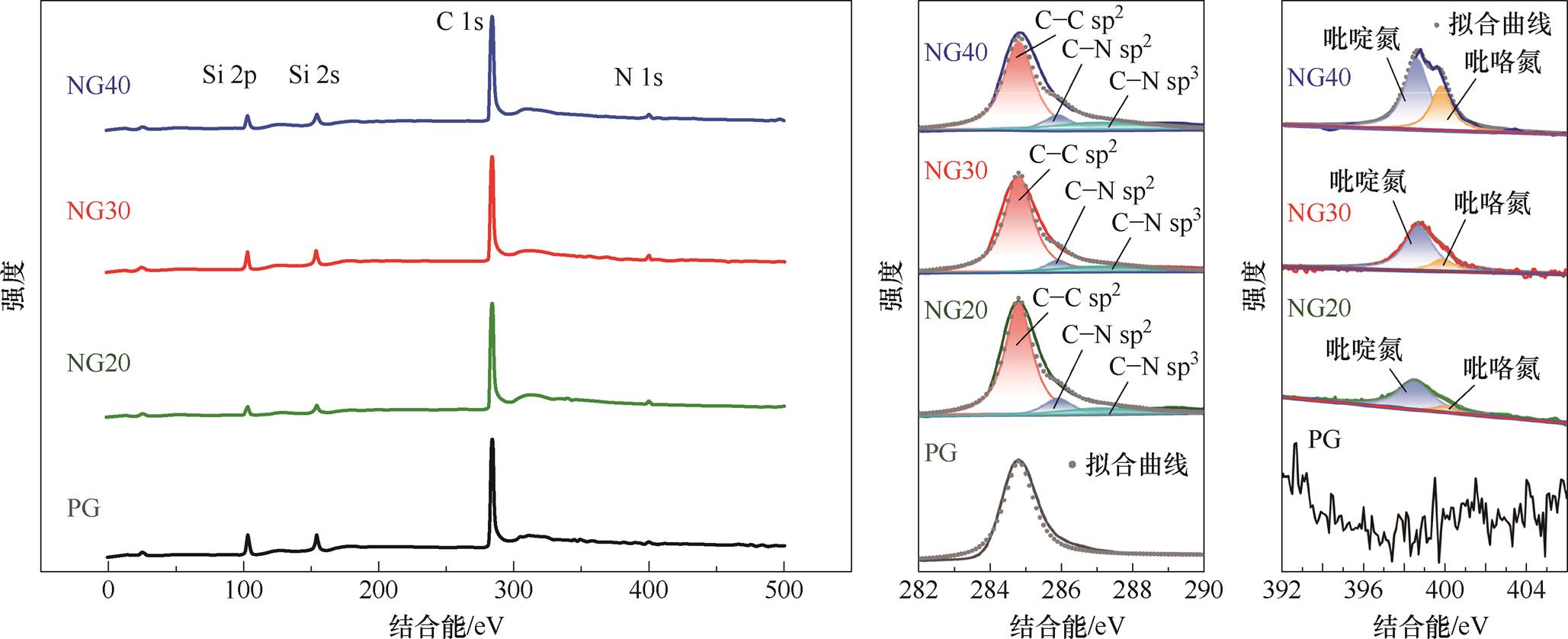
(a)全谱图(b)C 1s能谱(c)N 1s能谱
图13 氮掺杂石墨烯XPS能谱图
Fig.13 XPS energy spectrum of nitrogen-doped graphen
基于第一性原理计算不同掺杂氮原子下石墨烯- NH3吸附体系的能量与结构参数。图14所示为本征石墨烯与氮掺杂石墨烯的态密度图,吡啶氮掺杂石墨烯与吡咯氮掺杂石墨烯的费米能级(图14中0 eV)处靠近价带侧的态密度都发生了较大程度的变化。石墨烯-NH3吸附体系的能量与结构参数计算结果见表3。吡啶氮与NH3之间的吸附能为负且绝对值最大,为-0.26 eV,吸附距离最小,为2.637 Å,转移电荷量最大,为0.04 e;吡咯氮的吸附能绝对值最小,为-0.08 eV,吸附距离最远,为3.005 Å,转移电荷量最小,为0.02 e。此结果说明吡啶氮对NH3的吸附性强于吡咯氮,可增强石墨烯对NH3分子的吸附。
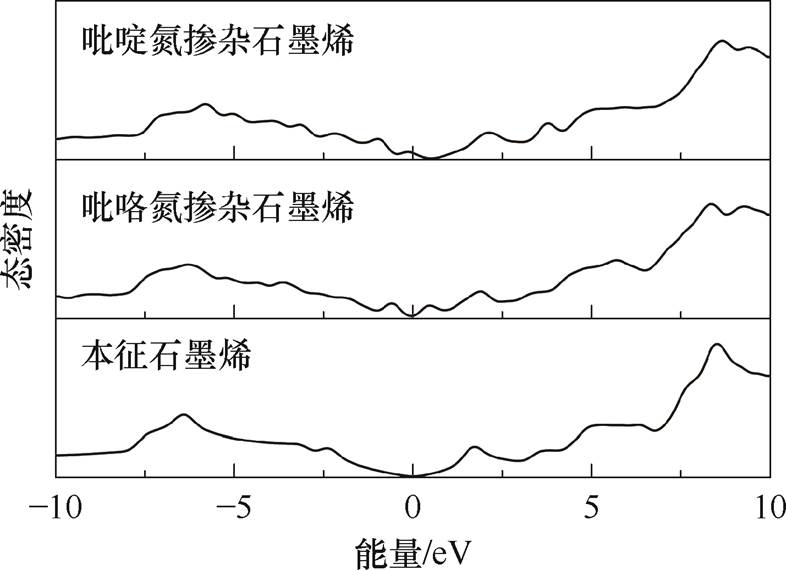
图14 石墨烯的态密度图
Fig.14 Density of states diagram of graphene
表3 石墨烯-NH3吸附体系的能量与结构参数
Tab.3 The energy and structural parameters of the graphene-NH3 adsorption system
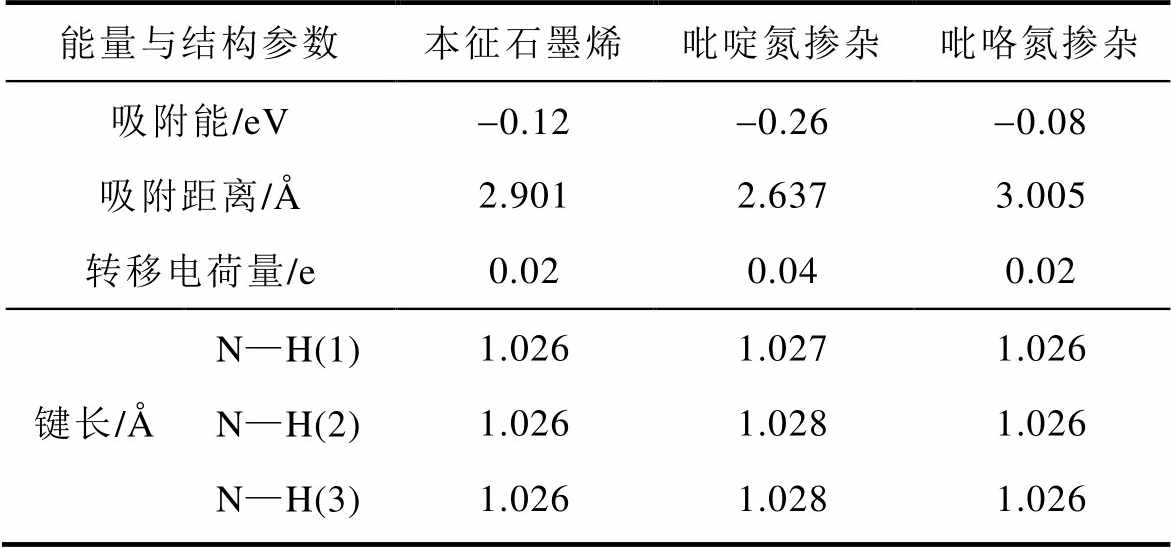
能量与结构参数本征石墨烯吡啶氮掺杂吡咯氮掺杂 吸附能/eV-0.12-0.26-0.08 吸附距离/Å2.9012.6373.005 转移电荷量/e0.020.040.02 键长/ÅN—H(1)1.0261.0271.026 N—H(2)1.0261.0281.026 N—H(3)1.0261.0281.026
注:1 Å=1×10-10 m, 1 e=1.602×10-19 C。
吡啶氮对NH3的吸附性能优于吡咯氮的原因可能与材料对NH3的响应机制有关。当传感器暴露在背景气氛围中时,氧气可以捕获材料中的电子形成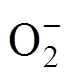 等离子吸附在表面,同时使石墨烯中的空穴浓度增加,呈p型半导体特性。NH3分子与这些离子反应释放电子,使石墨烯中空穴浓度降低,表现为传感器电阻升高,吸附反应过程如下。
等离子吸附在表面,同时使石墨烯中的空穴浓度增加,呈p型半导体特性。NH3分子与这些离子反应释放电子,使石墨烯中空穴浓度降低,表现为传感器电阻升高,吸附反应过程如下。
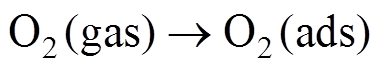 (4)
(4)
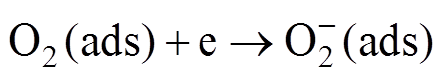 (5)
(5)
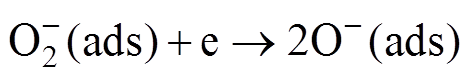 (6)
(6)
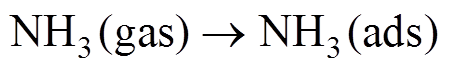 (7)
(7)
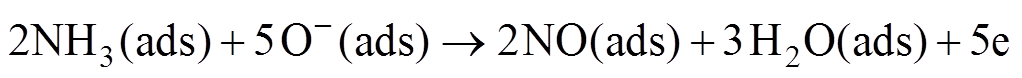 (8)
(8)
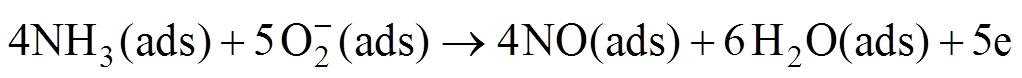 (9)
(9)
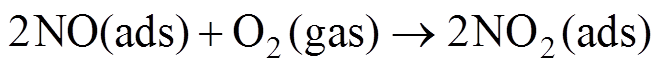 (10)
(10)
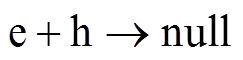 (11)
(11)
式中,gas代表该物质为气态;ads代表该物质为吸附态;null代表正负电荷中和。
由于在石墨烯晶格中,吡啶氮替代一个碳原子,与其他碳原子形成悬空六元环,其孤对电子不参与共轭体系,而是垂直于环平面,更容易与外部分子相互作用;吡咯氮则形成悬空五元环,孤对电子参与共轭体系,使其电子云分布更倾向于环内,与外部分子的相互作用能力相对较弱。故吡啶氮的高活性孤对电子更容易与氧气分子反应形成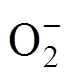 等离子,有利于NH3的吸附,表现出比吡咯氮更好的NH3吸附性能。
等离子,有利于NH3的吸附,表现出比吡咯氮更好的NH3吸附性能。
根据XPS表征结果,当氮掺杂浓度不高时,氮掺杂类型以吡啶氮为主;随着掺杂浓度的提升,吡啶氮的数量迅速上升,传感器对NH3的气敏性能也随着掺杂浓度的提高而极大提升,故NG30传感器的性能优于NG20传感器;随着掺杂浓度继续提高,虽然吡啶氮仍占主导,但吡咯氮的比例也从12.1%增加到41.9%,由于吡咯氮与NH3间的吸附能不高,传感器对NH3的气敏性能也随着氮掺杂浓度的提升而迅速下降,故NG40传感器的性能劣于NG30传感器。
本文制备了不同氮掺杂浓度的石墨烯基NH3传感器并进行了表征与气敏测试,研究了氮掺杂浓度对传感器性能的影响机理,具体结论如下:
1)石墨烯的氮掺杂浓度随尿素用量增大而提升;当尿素用量小于30 mg即掺杂浓度较低时,石墨烯中掺杂氮原子主要为吡啶氮;当掺杂浓度升高时,吡咯氮相对含量增大。文中传感器的吡啶氮含量大小关系为:NG30>NG20>NG40。
2)NG30传感器的性能优于掺杂浓度更高的NG40传感器。NG30传感器对体积分数为100×10-6的NH3的响应为11.7%,选择性系数最大为80,灵敏度为0.110 0%/10-6,最低检测限为71×10-9,响应/恢复时间为373/568 s,重复性与长期稳定性良好。
3)吡啶氮与NH3之间的吸附能为-0.26 eV,吸附距离为2.637 Å,转移电荷量为0.04 e,吸附性强于本征石墨烯;吡咯氮与NH3之间的吸附能为-0.08 eV,吸附距离为3.005 Å,转移电荷量为0.02 e,吸附性弱于本征石墨烯。NG30掺杂原子主要为吡啶氮,可增强石墨烯对NH3的吸附,提高传感器性能;而NG40吡咯氮含量较高,吸附性减弱,传感器性能降低。掺杂浓度通过改变石墨烯中吡啶氮与吡咯氮的比例来影响其性能,氮掺杂石墨烯基NH3传感器的性能随吡啶氮含量的增加而增强,随吡咯氮含量的增加而劣化。
参考文献
[1] Giddey S, Badwal S P S, Munnings C, et al. Ammonia as a renewable energy transportation media[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(11): 10231-10239.
[2] Mukherjee S, Devaguptapu S V, Sviripa A, et al. Low-temperature ammonia decomposition catalysts for hydrogen generation[J]. Applied Catalysis B: Environmental, 2018, 226: 162-181.
[3] 吴全, 沈珏新, 余磊, 等.“双碳”背景下氢-氨储运技术与经济性浅析[J]. 油气与新能源, 2022, 34(5): 27-33, 39.
Wu Quan, Shen Juexin, Yu Lei, et al. Analysis on the hydrogen-ammonia storage and transportation techno- logy and economical efficiency against the “dual- carbon” background[J]. Petroleum and New Energy, 2022, 34(5): 27-33, 39.
[4] Lee I, Jung B, Park J, et al. Mixed potential NH3 sensor with LaCoO3 reference electrode[J]. Sensors and Actuators B: Chemical, 2013, 176: 966-970.
[5] 祁福平, 党红娟. 化工企业氨气含量检测方法简评[J]. 中氮肥, 2022(5): 69-72.
[6] 傅文翔, 董力强, 杨柳. 光声光谱法检测化学毒剂模拟剂及毒害气体研究进展[J]. 光谱学与光谱分析, 2023, 43(12): 3653-3658.
Fu Wenxiang, Dong Liqiang, Yang Liu. Research progress on detection of chemical warfare agent simulants and toxic gases by photoacoustic spectros- copy[J]. Spectroscopy and Spectral Analysis, 2023, 43(12): 3653-3658.
[7] 吴军, 田学航. 光声光谱技术与气相色谱技术在变压器在线监测中的分析比较[J]. 电气技术, 2013, 14(12): 65-68.
Wu Jun, Tian Xuehang. Analysis and compare of photo-acoustic spect roscopy technology and gas chromatography technology in transformer on-line detecting[J]. Electrical Engineering, 2013, 14(12): 65-68.
[8] Ji Xiaobo, Banks C E, Compton R G. The electroche- mical oxidation of ammonia at boron-doped diamond electrodes exhibits analytically useful signals in aqueous solutions[J]. Analyst, 2005, 130(10): 1345- 1347.
[9] 熊华竞, 苏丹, 王维康. 室内环境甲醛气体检测技术的研究进展[J]. 化学分析计量, 2024, 33(10): 127- 136.
Xiong Huajing, Su Dan, Wang Weikang. Research development of formaldehyde gas detection technology in indoor environment[J]. Chemical Analysis and Meterage, 2024, 33(10): 127-136.
[10] 江军, 张文乾, 李波, 等. 电力变压器油中溶解气体离群值识别和数据重构[J]. 电工技术学报, 2024, 39(17): 5521-5533.
Jiang Jun, Zhang Wenqian, Li Bo, et al. Outlier detection and data reconstruction of dissolved gas in oil for power transformers[J]. Transactions of China Electrotechnical Society, 2024, 39(17): 5521-5533.
[11] 向英瀚, 柏博旭, 侯士波, 等. 基于SnS2传感器的H2S和SO2F2气体检测性能研究[J]. 高压电器, 2023, 59(3): 132-139.
Xiang Yinghan, Bai Boxu, Hou Shibo, et al. Research on H2S and SO2F2 gas detection performance based on SnS2 sensor[J]. High Voltage Apparatus, 2023, 59(3): 132-139.
[12] 杨梦洁, 杨爱军, 叶奕君, 等. 基于气体分析的锂离子电池热失控早期预警研究进展[J]. 电工技术学报, 2023, 38(17): 4507-4538.
Yang Mengjie, Yang Aijun, Ye Yijun, et al. Research progress on early warning of thermal runaway of Li-ion batteries based on gas analysis[J]. Transactions of China Electrotechnical Society, 2023, 38(17): 4507-4538.
[13] 孟国栋, 李雨珮, 唐佳, 等. 锂离子电池储能电站的热失控状态检测与安全防控技术研究进展[J]. 高电压技术, 2024, 50(7): 3105-3127.
Meng Guodong, Li Yupei, Tang Jia, et al. Research progress of thermal runaway detection and safety control technology for lithium-ion battery energy storage power stations[J]. High Voltage Engineering, 2024, 50(7): 3105-3127.
[14] 第五华婷, 葛万银, 赵虎, 等. Sn掺杂ZIF-8衍生ZnO纳米材料的制备及其NO2传感性能[J]. 陕西科技大学学报, 2024, 42(5): 126-133.
Diwu Huating, Ge Wanyin, Zhao Hu, et al. Prepara- tion of Sn-doped ZIF-8-derived ZnO nanomaterials and their NO2 sensing properties[J]. Journal of Shaanxi University of Science & Technology, 2024, 42(5): 126-133.
[15] 吴晓芳, 王晓雨, 黄宝玉, 等. 基于SnO2/SnTe材料的氨气传感器性能[J]. 微纳电子技术, 2023, 60(9): 1445-1456.
Wu Xiaofang, Wang Xiaoyu, Huang Baoyu, et al. Performance of ammonia sensor based on SnO2/SnTe material[J]. Micronanoelectronic Technology, 2023, 60(9): 1445-1456.
[16] 张冬至, 姜传星, 殷乃良, 等. 纳米修饰石墨烯薄膜传感器的变压器油中乙炔气体敏感特性[J]. 高电压技术, 2017, 43(11): 3712-3717.
Zhang Dongzhi, Jiang Chuanxing, Yin Nailiang, et al. Sensing characteristics of acetylene gas in trans- former oil of nano-modified graphene-based film sensor[J]. High Voltage Engineering, 2017, 43(11): 3712-3717.
[17] 庞思远, 刘希喆. 石墨烯在电气领域的研究与应用综述[J]. 电工技术学报, 2018, 33(8): 1705-1722.
Pang Siyuan, Liu Xizhe. Review on research and application of graphene in electrical field[J]. Trans- actions of China Electrotechnical Society, 2018, 33(8): 1705-1722.
[18] 杨丽, 陈雪, 王子涵, 等. 基于激光诱导石墨烯的柔性超疏水温度传感器研究[J]. 电工技术学报, 2023, 38(增刊1): 257-266.
Yang Li, Chen Xue, Wang Zihan, et al. Research on flexible and superhydrophobic temperature sensor based on laser-induced graphene[J]. Transactions of China Electrotechnical Society, 2023, 38(S1): 257- 266.
[19] 高新, 李志慧, 刘宇鹏, 等. 改性石墨烯基传感器对SF6分解组分H2S的吸附机理及检测特性研究[J]. 电工技术学报, 2023, 38(13): 3606-3618.
Gao Xin, Li Zhihui, Liu Yupeng, et al. Study on adsorption mechanism and detection characteristics of modified graphene sensors for SF6 decomposed component H2S[J]. Transactions of China Electrotech- nical Society, 2023, 38(13): 3606-3618.
[20] Zhang Li, Li Chun, Liu Anran, et al. Electrosynthesis of graphene oxide/polypyrene composite films and their applications for sensing organic vapors[J]. Journal of Materials Chemistry, 2012, 22(17): 8438- 8443.
[21] Lü Ruitao, Chen Gugang, Li Qing, et al. Ultrasensi- tive gas detection of large-area boron-doped graphene [J]. Proceedings of the National Academy of Sciences of the United States of America, 2015, 112(47): 14527-14532.
[22] Srivastava S, Kashyap P K, Singh V, et al. Nitrogen doped high quality CVD grown graphene as a fast responding NO2 gas sensor[J]. New Journal of Chemistry, 2018, 42(12): 9550-9556.
[23] Kim Y H, Park J S, Choi Y R, et al. Chemically fluorinated graphene oxide for room temperature ammonia detection at ppb levels[J]. Journal of Materials Chemistry A, 2017, 5(36): 19116-19125.
[24] 郑钦仁, 詹涪至, 折俊艺, 等. 石墨烯的形貌特征对其场发射性能的影响[J]. 物理学报, 2024, 73(8): 216-225.
Zheng Qinren, Zhan Fuzhi, She Junyi, et al. Influence of morphological characteristics of graphene on its field emission properties[J]. Acta Physica Sinica, 2024, 73(8): 216-225.
[25] 吴天如. 化学气相沉积法生长高质量石墨烯及其光电性能研究[D]. 南京: 南京航空航天大学, 2012.
Wu Tianru. Investigation on fabrication and optoelec- tronic properties of high-quality graphene grown by CVD[D]. Nanjing: Nanjing University of Aeronautics and Astronautics, 2012.
[26] Deokar G, Jin Junjie, Schwingenschlögl U, et al. Chemical vapor deposition-grown nitrogen-doped graphene’s synthesis, characterization and applications [J]. NPJ 2D Materials and Applications, 2022, 6: 14.
[27] 王琼苑, 褚继峰, 李秋霖, 等. 基于微型气体传感阵列的空气绝缘设备放电故障识别[J]. 电工技术学报, 2023, 38(23): 6494-6502.
Wang Qiongyuan, Chu Jifeng, Li Qiulin, et al. Miniature gas-sensing array employed for the discharge fault diagnosis of air-insulated equipment[J]. Trans- actions of China Electrotechnical Society, 2023, 38(23): 6494-6502.
[28] Perdew J, Burke K, Ernzerhof M. Generalized gradient approximation made simple[J]. Physical Review Letters, 1996, 77(18): 3865-3868.
[29] Zhang Yonghui, Chen Yabin, Zhou Kaige, et al. Improving gas sensing properties of graphene by introducing dopants and defects: a first-principles study[J]. Nanotechnology, 2009, 20(18): 185504.
[30] Wang Xinran, Li Xiaolin, Zhang Li, et al. N-doping of graphene through electrothermal reactions with ammonia[J]. Science, 2009, 324(5928): 768-771.
[31] Zhang Chaohua, Fu Lei, Liu Nan, et al. Synthesis of nitrogen-doped graphene using embedded carbon and nitrogen sources[J]. Advanced Materials, 2011, 23(8): 1020-1024.
[32] 田甜, 吕敏, 田旸, 等. 石墨烯的生物安全性研究进展[J]. 科学通报, 2014, 59(20): 1927-1936.
Tian Tian, Lü Min, Tian Yang, et al. Progress in biological safety of graphene[J]. Chinese Science Bulletin, 2014, 59(20): 1927-1936.
[33] 姚雅萱, 任玲玲, 高思田, 等. 石墨烯层数测量方法的研究进展[J]. 化学通报, 2015, 78(2): 100-106.
Yao Yaxuan, Ren Lingling, Gao Sitian, et al. Progress in measuremental methods for layer numbers of graphene[J]. Chemistry, 2015, 78(2): 100-106.
[34] Lherbier A, Blase X, Niquet Y M, et al. Charge transport in chemically doped 2D graphene[J]. Physical Review Letters, 2008, 101(3): 036808.
[35] Sharma A K, Mahajan A. Potential applications of chemiresistive gas sensors[M]//Dhall S. Carbon Nanomaterials and Their Nanocomposite-Based Chemiresistive Gas Sensors. Amsterdam: Elsevier, 2023: 223-245.
[36] Wusiman M, Taghipour F. Methods and mechanisms of gas sensor selectivity[J]. Critical Reviews in Solid State and Materials Sciences, 2022, 47(3): 416-435.
[37] Tualeka A R. Procedures determination of chemical contaminants limit gas safe working environment (case studies on ammonia)[J]. Indonesian Journal of Occupational Safety and Health, 2013, 1(1): 3800.
[38] Baier E J. NIOSH testimony to DOL: Occupational Safety and Health Administration public hearing on an occupational standard for sulfur dioxide[R]. Washington D. C.: the US Department of Labor, 1977.
[39] Afshar-Mohajer N, Zuidema C, Sousan Sinan, et al. Evaluation of low-cost electro-chemical sensors for environmental monitoring of ozone, nitrogen dioxide, and carbon monoxide[J]. Journal of Occupational and Environmental Hygiene, 2018, 15(2): 87-98.
[40] 住房和城乡建设部, 国家质量监督检验检疫总局. 石油化工可燃气体和有毒气体检测报警设计规范: GB 50493—2009[S]. 北京: 中国计划出版社, 2009.
[41] Lee Y, Lee S, Hwang Y, et al. Modulating magnetic characteristics of Pt embedded graphene by gas adsorption (N2, O2, NO2, SO2)[J]. Applied Surface Science, 2014, 289: 445-449.
[42] Lu Ganhua, Ocola L E, Chen Junhong. Reduced graphene oxide for room-temperature gas sensors[J]. Nanotechnology, 2009, 20(44): 445502.
[43] Hayasaka T, Kubota Y, Liu Yumeng, et al. The influences of temperature, humidity, and O2 on electrical properties of graphene FETs[J]. Sensors and Actuators B: Chemical, 2019, 285: 116-122.
[44] Joshi R K, Gomez H, Alvi F, et al. Graphene films and ribbons for sensing of O2, and 100 ppm of CO and NO2 in practical conditions[J]. The Journal of Phy- sical Chemistry C, 2010, 114(14): 6610-6613.
[45] 赵珉, 褚卫国, 刘桂英, 等. 石墨烯/铜异质结室温气体传感器性能研究[J]. 传感技术学报, 2021, 34(12): 1615-1621.
Zhao Min, Chu Weiguo, Liu Guiying, et al. Study on the performances of graphene/copper heterojunction room temperature gas sensor[J]. Chinese Journal of Sensors and Actuators, 2021, 34(12): 1615-1621.
[46] Hoa N D, An S Y, Dung N Q, et al. Synthesis of p-type semiconducting cupric oxide thin films and their application to hydrogen detection[J]. Sensors and Actuators B: Chemical, 2010, 146(1): 239-244.
[47] Odebowale A A, Abdulghani A, Berhe A M, et al. Emerging low detection limit of optically activated gas sensors based on 2D and hybrid nanostructures[J]. Nanomaterials, 2024, 14(18): 1521.
[48] 漆红兰,李佳妮, 张成孝. 检出限与灵敏度关系及影响因素的探讨[J]. 大学化学, 2021, 36(9): 219-225.
Qi Honglan, Li Jiani, Zhang Chengxiao. Discussion on the relationship and influence factors of the limit of detection and the sensitivity of analytical methods [J]. University Chemistry, 2021, 36(9): 219-225.
[49] 刘志华, 刘琭琭, 韩丹. 二维材料Ti3C2Tx MXene对氨气的气敏性能[J]. 微纳电子技术, 2023, 60(1): 70-77.
Liu Zhihua, Liu Lulu, Han Dan. Sensing properties of two-dimensional material Ti3C2Tx MXene to ammonia[J]. Micronanoelectronic Technology, 2023, 60(1): 70-77.
[50] Liu Lili, Qing Miaoqing, Wang Yibo, et al. Defects in graphene: generation, healing, and their effects on the properties of graphene: a review[J]. Journal of Mate- rials Science & Technology, 2015, 31(6): 599- 606.
[51] Wu Tianru, Shen Honglie, Sun Lei, et al. Nitrogen and boron doped monolayer graphene by chemical vapor deposition using polystyrene, urea and boric acid[J]. New Journal of Chemistry, 2012, 36(6): 1385- 1391.
Abstract Ammonia (NH3), with a hydrogen storage capacity of 17.7%, is considered a promising hydrogen energy carrier under the goal of dual-carbon(carbon neutrality and carbon peaking). However, due to its high volatility and toxicity, the online detection of NH3 is essential for safe ammonia-hydrogen storage and transport. Gas sensors based on gas-sensitive materials are widely used for NH3 detection, but intrinsic graphene exhibits weak physical adsorption to most gases, limiting its sensing performance. Doping, functionalization, and compositing are effective strategies for improving graphene-based gas sensors. However, the effect of different nitrogen doping concentrations on NH3 sensing performance remains underexplored.
Firstly, graphene sensors with different nitrogen doping (NG) concentrations were prepared, and their morphology, structure and chemical composition were systematically characterized. The influence of nitrogen doping concentration on the structural properties of graphene was analyzed. Secondly, the gas sensing response of sensors with different doping concentrations was tested, and the influence of doping concentration on sensor performance was analyzed. Finally, the energy and structural parameters of the nitrogen-doped graphene-NH3 adsorption system were calculated based on the first-principles, and the influence mechanism of doping concentration on gas sensing performance was discussed.
The gas sensitivity tests show that the NG30 sensor exhibits optimal performance, with an 11.7% response, a maximum selectivity coefficient of 80, a sensitivity of 0.11%/10-6, a minimum detection limit of 71×10-9, and response/recovery times of 373/568 s. Long-term exposure tests showed that NG sensors maintained over 90% of their initial response even after multiple NH3 exposure cycles, demonstrating excellent stability and repeatability. Computational simulations indicate that when the nitrogen content in graphene is below 1.44%, pyridine nitrogen dominates, resulting in stronger NH3 adsorption with an adsorption energy of -0.26 eV, an adsorption distance of 2.637 Å (1 Å=1×10-10 m), and a charge transfer of 0.04 e (1 e=1.602×10-19 C). This enhanced adsorption improves sensor performance. In contrast, when the nitrogen content exceeds 1.44%, pyrrole nitrogen becomes dominant, leading to weaker adsorption with an adsorption energy of -0.08 eV, an adsorption distance of 3.005 Å, and a charge transfer of 0.02 e, thereby deteriorating sensor performance. Further analysis reveals that nitrogen doping influences graphene’s electronic structure, altering its conductivity and adsorption characteristics. Density functional theory (DFT) calculations indicate that pyridine nitrogen contributes to a higher density of states near the Fermi level, facilitating charge transfer during NH3 adsorption. In contrast, pyrrole nitrogen introduces localized states that weaken adsorption interactions. The experimental findings align with theoretical predictions, confirming that optimal NH3 sensing performance is achieved at a nitrogen doping concentration of 1.44%.
The following conclusions can be drawn from the analysis: (1) When the doping concentration is low, the doped nitrogen atoms in graphene are mainly pyridine nitrogen. When the doping concentration increases, the relative content of pyrrole nitrogen increases. (2) If the nitrogen doping concentration is too high, the performance of the sensor will deteriorate. (3) Pyridine nitrogen can enhance the adsorption of graphene to NH3, while pyrrole nitrogen will weaken the adsorption. The performance of nitrogen-doped graphene-based NH3 sensor increases with the increase of pyridine nitrogen content, and deteriorates with the increase of pyrrole nitrogen content.
keywords:Graphene, nitrogen doping, doping concentration, first principles, ammonia sensor
DOI: 10.19595/j.cnki.1000-6753.tces.242396
中图分类号:TM23;TP212
中央高校基本科研业务费(xzy012023152, xtr062023001, xtr052025002)和陕西省创新能力支撑计划资金(2024RS-CXTD-22)资助项目。
收稿日期 2024-12-31
改稿日期 2025-02-24
王建宇 男,2003年生,博士研究生,研究方向为二维材料与器件应用、微纳尺度绝缘与放电等离子体。E-mail: 2203712763@stu.xjtu.edu.cn
孟国栋 男,1985年生,博士,教授,博士生导师,研究方向为二维材料与器件应用、微纳尺度绝缘与放电等离子体、电力设备绝缘状态评估及检测诊断技术等。E-mail: gdmengxjtu@xjtu.edu.cn(通信作者)
(编辑 李 冰)