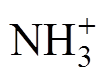 以及中性粒子NH和NH2,目标产物H2主要产生在电压高电平阶段的阴极位降区附近,研究结果可为优化制氢效果和确定最优参数提供指导。
以及中性粒子NH和NH2,目标产物H2主要产生在电压高电平阶段的阴极位降区附近,研究结果可为优化制氢效果和确定最优参数提供指导。摘要 等离子体具有工作温度低、电子能量范围分布宽等优势,已成为极具前景的氨气制氢技术,有效地调控等离子体对氨气高效制氢具有重要意义。该文采用方波脉冲电压驱动Ar-NH3介质阻挡放电(DBD),结合一维流体数值仿真研究了放电特性及产物分布。结果表明,一个放电周期内,在脉冲电压上升沿阶段,外施电压增加导致气体击穿,出现正电流脉冲;在脉冲电压下降沿阶段,自建电场主导产生负电流脉冲。放电演化过程呈现典型的辉光放电特性,电子密度、约化电场强度均在放电熄灭后的高电平起始阶段达到峰值,出现在阴极鞘层区附近。分析放电产物发现,主要粒子包括Ar*和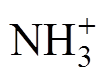 以及中性粒子NH和NH2,目标产物H2主要产生在电压高电平阶段的阴极位降区附近,研究结果可为优化制氢效果和确定最优参数提供指导。
以及中性粒子NH和NH2,目标产物H2主要产生在电压高电平阶段的阴极位降区附近,研究结果可为优化制氢效果和确定最优参数提供指导。
关键词:脉冲介质阻挡放电 氨气制氢 放电特性 放电产物
氨(NH3)的储氢密度高(氢的质量分数为17.6%),易液化、储运成本低,分解目标产物为H2和副产物N2,无碳氧化物排放,被认为是具有潜力、安全的氢替代能源载体。氨分解制氢反应为2NH3→3H2+N2(焓变DH=+46 kJ/mol),制氢过程吸热、能量需求高。催化热分解氨制氢技术转化效果较好,但运行温度高,反应有效裂解温度依赖所使用的催化剂(Ru、Ni、Fe和Co等),例如,镍基催化剂运行温度需要超过1 000℃,其他催化剂至少也需要650~700℃才能达到较为理想的转化水平[1-3]。同时,这种技术及装备还面临预热时间长、绝热系统庞大等问题,仍需研究人员开展深入系统的工作。因此,开发能够在较低温度下或替代工艺下满足氨分解制氢的新技术成为主要的探索方向。
放电等离子体已在材料处理、生物医学、环境化工等多个领域具有广泛的应用和研究[4-8]。等离子体中富含高能电子,且电子能量分布范围宽,呈非平衡分布特征,容易满足氨分解反应制氢的能量阈值和激活催化剂活性位点,可突破热力学反应限制,在高化学惰性分子(NH3、CO2、苯类等)活化、裂解方面表现出独特优势,成为近年来非热分解氨制氢的潜力股和研究热点[9-12]。
在氨气(或含氨)氛围中,kHz级正弦电压、微波和射频驱动的放电已有相关实验和数值仿真研究,使人们对氨气放电等离子体有较为深入的理解。张增辉等[13]研究了大气压下正弦电压驱动Ar/NH3介质阻挡辉光放电的放电机理,得到了气体间隙电压、介质表面电荷密度、放电电流密度的时间周期变化,以及带电粒子、中性粒子与空间电场强度的时空分布。M. El-Shafie等[14]通过实验比较了正弦驱动介质阻挡放电(Dielectric Barrier Discharge, DBD)等离子体分解氨的三个制氢系统的能量和火用效率,分析了不同氨输入流量下的氨分解反应器系统性能。R. A. Arakoni等[15]利用二维模型研究了Ar/NH3混合气体微放电产生H2,得出在最优条件下可达到83%的转化效率。微波主要利用高频电磁波能量驱动放电等离子体。Li Zhi等[16]采用二维流体模型研究了低压Ar/NH3微波电子回旋共振放电等离子体的特性;Niu Yulong等[17]在常压下使用氮气微波等离子体炬的余辉分解NH3制氢,研究了旋转温度和激发物质浓度随微波功率和NH3流速的变化,讨论了等离子体余辉中NH3的分解机制;Zhang Xinhua等[18]研究了微波等离子体射流催化氨裂解制氢,以氩气为载气,开展了大气压下不同的微波输入功率、总气体流量和NH3初始含量的实验,发现产氢速率随微波功率和NH3含量的增加呈线性增长,与总流量基本无关;并通过建立简化的一维热反应模型,分析了等离子体化学中热分解NH3制氢的重要性。与微波放电相比,射频电源驱动等离子体有放电更加稳定等优点。R. Bazinette等[19]研究了电压幅值和频率对Ar-NH3彭宁混合气体在双频电压驱动下的汤森、辉光和射频DBD的影响;R. Robert等[20-21]基于Ar-NH3混合物的平板DBD实验和数值模型,研究了常压下双频(5 MHz/ 50kHz)DBD从a模式到a-g模式的转变,并利用发射和吸收光谱研究了Ar-NH3 DBD产物的时间演化。脉冲电压具有陡上升/下降沿和窄脉宽等特点,电压变化速率快,增强了激发和电离过程,有助于形成均匀稳定的等离子体区[22-23]。目前围绕多种气体氛围中的等离子体放电特性及应用开展了大量研究,充分体现了微/纳秒脉冲放电在改善放电均匀性、提升粒子丰度等方面的优越性[24-29]。而在氨气放电研究方面,尚未见相关报道。
本文采用平行平板介质阻挡放电电极结构,结合实验和一维流体仿真模型,研究脉冲电压驱动的Ar-NH3 DBD特性、基本参数和产物分布,以期为等离子体氨制氢的研究及应用积累数据和提供帮助。
本文采用的实验平台如图1所示,详细结构见团队前期发表的成果[30-31]。实验在常温常压下进行,放电单元为双介质阻挡放电(DBD)结构。DBD单元的上电极通过高压套管与脉冲电源连接,下电极通过真空腔壁的接地套管引出后独立接地,电极和阻挡介质用导电胶粘接。阻挡介质为相对介电常数为3.7的石英,阻挡介质的直径均为40 mm,厚度d1=d2=1 mm,气隙距离dg=4 mm。工作气体为Ar和NH3混合气体,对反应器抽真空后通入混合气体使NH3占比(体积分数)为0.5%,且保持总压强为100 kPa。放电驱动电压为上升沿(tr)和下降沿(tf)时间均为100 ns、脉冲宽度(tw)为1 000 ns、重复频率(f)为20 kHz的脉冲波形。电压和电流信号分别由高压探头(Tektronix P6015A)和电流线圈(Pearson 6585)监测,采集信号由数字示波器(Tek DSO 34)显示并存储。采用ICCD(intensified charge coupled device)相机(Andor, iStar DHT-334)和中阶梯光谱仪(Mechelle 5000)分别拍摄其演化行为和发射光谱。
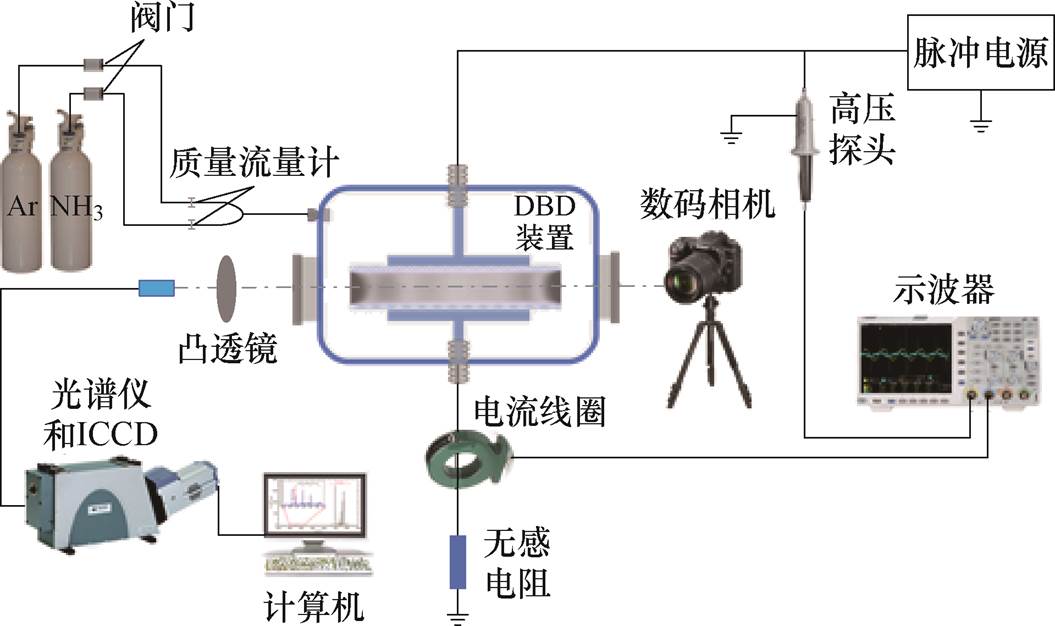
图1 实验平台
Fig.1 Experimental platform
为了解释文中所述实验放电过程及产物分布特性,搭建了一维流体仿真模型,对应电极结构仿真模型如图2所示。仿真中的参数设置与实验一致。两块金属板作为电极,表面覆盖石英阻挡介质,其直径为50 mm、厚度d1=d2=1 mm,相对介电常数er1= er2=3.7,气体间隙dg=4 mm,气隙相对介电常数er0=1。纳秒脉冲外施电压幅值ua=3 kV,重复频率f= 20 kHz,脉冲宽度tw=1 000 ns,脉冲上升沿和下降沿tr=tf =100 ns,Ar-NH3混合气体中NH3体积分数为0.5%。
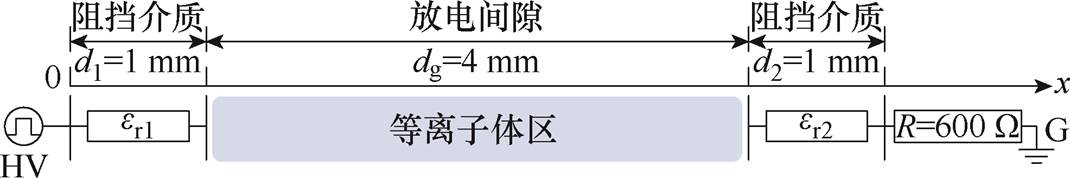
图2 仿真模型
Fig.2 Simulation model
模型中通过描述电子、离子和中性粒子的一维连续性方程,并结合泊松方程求解放电区域的电场。由于气体间隙远小于电极直径,假设文中介质阻挡放电沿径向均匀分布,因此,可以采用一维模型探讨放电发展过程的特性和参数变化。详细的模型描述,包括具体数学描述、考虑的反应以及粒子类型和边界条件等,参见作者前期发表的成果[32-33]。
各粒子数量守恒方程,即一维连续性方程[34]为
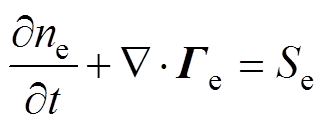 (1)
(1)
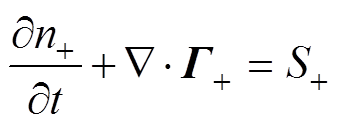 (2)
(2)
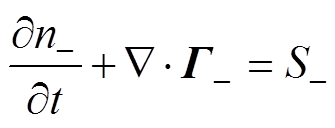 (3)
(3)
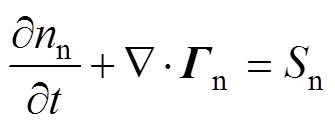 (4)
(4)
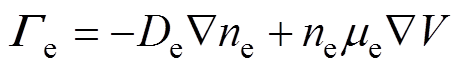 (5)
(5)
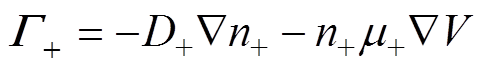 (6)
(6)
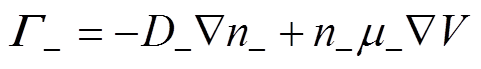 (7)
(7)
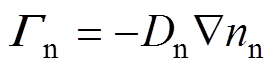 (8)
(8)
式中,下角标e、+、-、n分别代表电子、正离子、负离子和中性粒子;n为相应的粒子数密度;G为相应的粒子通量;S为相应的粒子源项;m为相应的粒子迁移率;D为相应的中性粒子扩散系数;V为外施电压。
电场采用泊松方程计算,表达式为
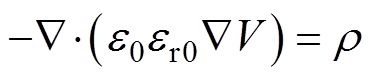 (9)
(9)
式中,e0为真空介电常数;r为空间电荷密度,表达式为
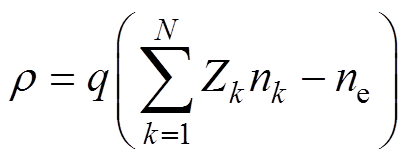 (10)
(10)
式中,q为元电荷量;Zk为离子k的电荷数;nk为离子k的数密度;N为离子种类数。
实验测量所得电压电流波形如图3a、图3b所示,图3c为仿真电压电流与表面电荷的变化波形。单个周期内的脉冲激励电压波形包括上升沿(tr)、脉宽(tw)以及下降沿(tf)三部分,分析图3a和图3b可知,电流波形较电压波形相位略超前,为容性放电,且实验中的电压电流波形受电路阻尼影响出现较大振荡,但实验与仿真的放电电压电流波形相似,可以满足本文研究需求。在单个周期的放电过程中,脉冲上升沿(A点)出现了正电流脉冲,且在电压极性未翻转的情况下,由于电压变化产生的电位差和表面电荷累积共同作用,在下降沿(D点)产生了二次放电电流脉冲。
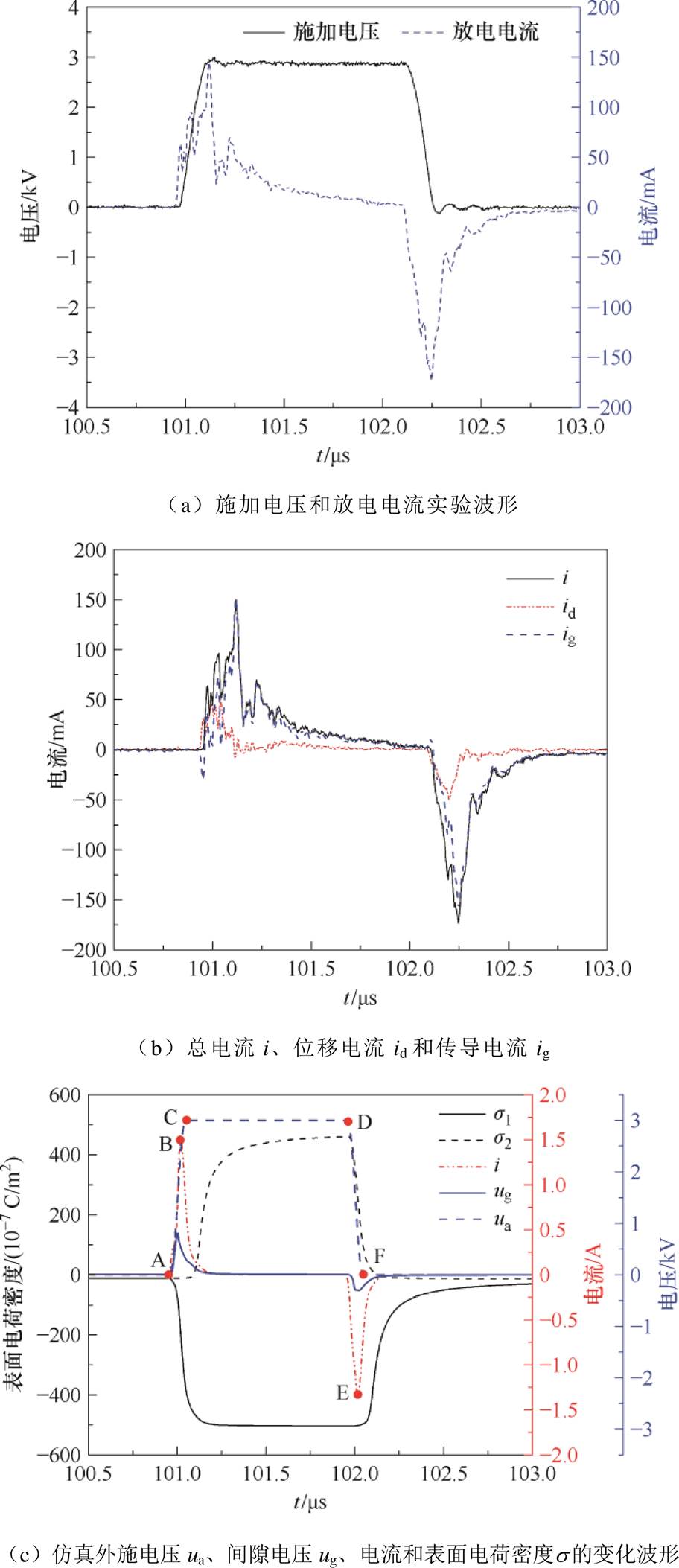
图3 基本电学特性参数分布
Fig.3 Distribution of basic electrical characteristics parameters
在外施电压上升沿阶段,气隙电压(ug)随着外施电压的增加而迅速增加,气体间隙中的负电荷(以电子为主)在电场的作用下向高压侧运动,反应产生的正离子向接地侧运动,当ug达到气体击穿阈值时发生放电,B点时刻正电流脉冲达到峰值。由于电子对电场的响应更快,因此优先在高压侧介质表面不断聚集,负表面电荷密度s1增加;正离子运动相对较慢,到达介质表面时间滞后,短时间内形成较弱的反向电场,外施电场主导间隙中的电离过程,促进放电起始并发展直至击穿,放电电流达到峰值。随着放电进行,负电荷粒子(以负离子为主)和正离子持续在介质表面积累并形成稳定的表面电荷,自建电场显著增大,导致间隙中净电场快速减小,同时,外施电压不断增强直至上升沿结束(C点),外施电场达到最大值并保持不变。脉冲平顶期电压幅值不变,相当于直流电压,由于DBD装置呈容性,当间隙电压降低至零时,放电一直处于熄灭状态。当外施电压脉冲进入下降阶段(D-F阶段),外施电场快速减小,电子快速向接地侧介质表面迁移并与原来积累的正表面电荷中和,快速削弱了正表面电荷密度s2,如图3c所示,表现为s2曲线下降沿更陡;而间隙中正离子缓慢迁移至高压侧介质表面,中和积累的负表面电荷,使s1缓慢减小(如图3c所示,表现为s1曲线下降沿更缓),进而造成间隙中净电场以自建电场为主,并显著增强,导致负放电发生,E点时刻出现负放电峰值。
根据2.1节中的放电电流、电压特性,利用ICCD对正放电演化过程的放电图像进行研究。ICCD拍摄门宽为10 ns,拍摄步长为10 ns,每幅累积20次,增益设置为4 095,放电图像及采集时刻如图4所示。正放电演化过程如图4a所示,放电起始时刻高压电极侧介质附近出现光强微弱的亮层,放电区域较小(如100.96~101.00 ms的放电图像),放电处于起始阶段,间隙中电场强度较小,在高压电极侧介质表面附近产生微弱的发光层,放电电流较小,光强分布符合汤森放电特征。随着时间推移,放电由高压电极侧向地电极侧发展(如101.00~101.05 ms的放电图像),最强发光区域逐渐向间隙中间移动,最终在地电极侧介质表面附近产生负辉区,同时在间隙中观察到法拉第暗区和正柱区,此时光强分布符合辉光放电特征[34-36]。整个放电过程实现从汤森模式向辉光模式的转变。外施电压进入平顶期后,放电光强达到最大值(如101.07~101.11 ms的放电图像),随着电流衰减和气隙电压降低,放电强度逐渐减弱直至熄灭,熄灭过程持续时间较长。图4b为负放电演化过程的实验图像,在高压侧缓慢形成发光层(如101.96~102.00 ms的放电图像),空间电子迅速向瞬时阳极移动,正离子向瞬时阴极移动,与正放电方向相反。随着时间推移,放电强度逐渐增强,达到峰值(102.07~102.11 ms),然后放电强度逐渐降低直至熄灭。与正放电相比,负放电在负辉区附近出现明显的发光夹层,说明电子在迁移过程中碰撞原子或分子发生的激发和电离过程较正放电更为剧烈。

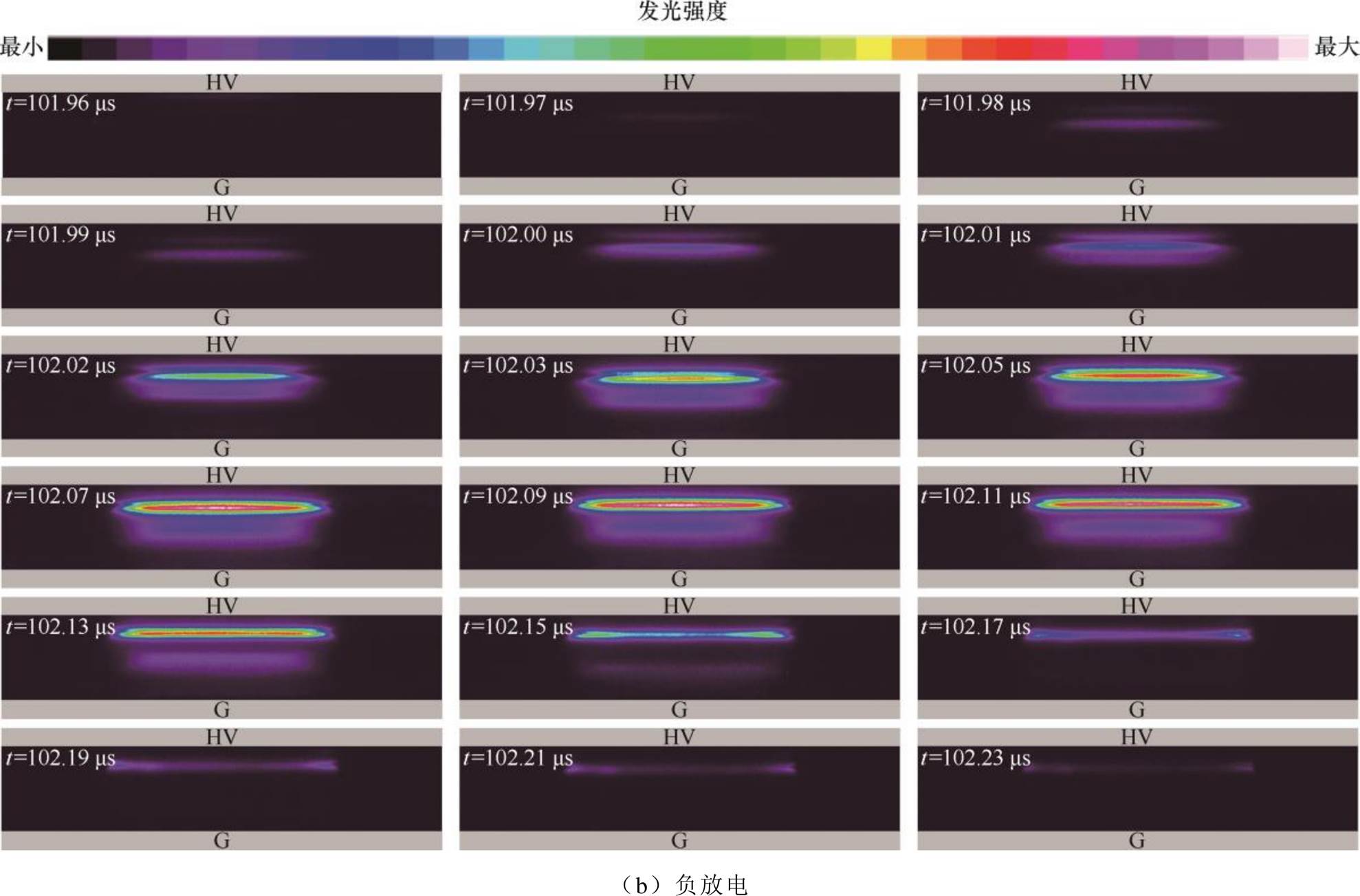
图4 短曝光放电演化行为
Fig.4 Discharge evolution behavior with short-time exposure
利用数值仿真分析一个电压脉冲期间电子密度和约化电场强度(E/n,其中E为电场强度,n为中性粒子数密度,单位为Td,1 Td=1×10-21 V·m2)的时空分布,如图5a和图5b所示。结合图4所示放电演化过程发现,在正放电起始阶段,间隙内电子及二次电子在电场中易获得足够多能量,在阴极位降区边界附近发生电离反应,产生大量电子,电子密度出现峰值;而正离子不断地向接地侧移动,主导了阴极位降区的离子分布,使约化电场强度在该区域变化最大,粒子的激发退激剧烈,呈现典型的辉光放电特性,与实验现象吻合。随着放电的发展,电子密度、约化电场强度均在上升沿末端之后达到最大值,如图5c所示。图5c中,各参数的下标A~F对应图3c中的A~F点。曲线A-C为上升沿阶段,放电电流在B点时刻达到峰值,随着上升沿电压的增加,接地侧电子密度、约化电场强度不断增强,两者的峰值在t=101.10 ms时刻达到最大。由于放电过程中电子的产生和复合反应,在上升沿阶段之后,电子密度持续上升并达到最大值。与实验结果相吻合,光强峰值也出现在上升沿之后的高电平阶段。
在高电平阶段,电子密度与约化电场强度峰值达到最大值后逐渐下降,电子密度维持在较低水平。而在下降沿阶段,外施电压剧烈变化,自建电场主导间隙发生反向放电,此时电子密度、约化电场强度的峰值在高压侧介质表面附近达到最大,过程与正放电相似,不再赘述。

图5 脉冲期间电子密度和约化电场强度分布
Fig.5 The spatiotemporal distribution of electron density and reduced electric field strength during the pulse period
本文实验采集了Ar-NH3 DBD的光学发射光谱,如图6所示。通过光谱可诊断出近紫外至近红外波段的部分放电产物,其中NH(光谱带头为324 nm、336 nm)和NH2(光谱范围为500~650 nm)以及Ar(696.5 nm)是Ar-NH3 DBD反应中的关键中间粒子[37-39]。
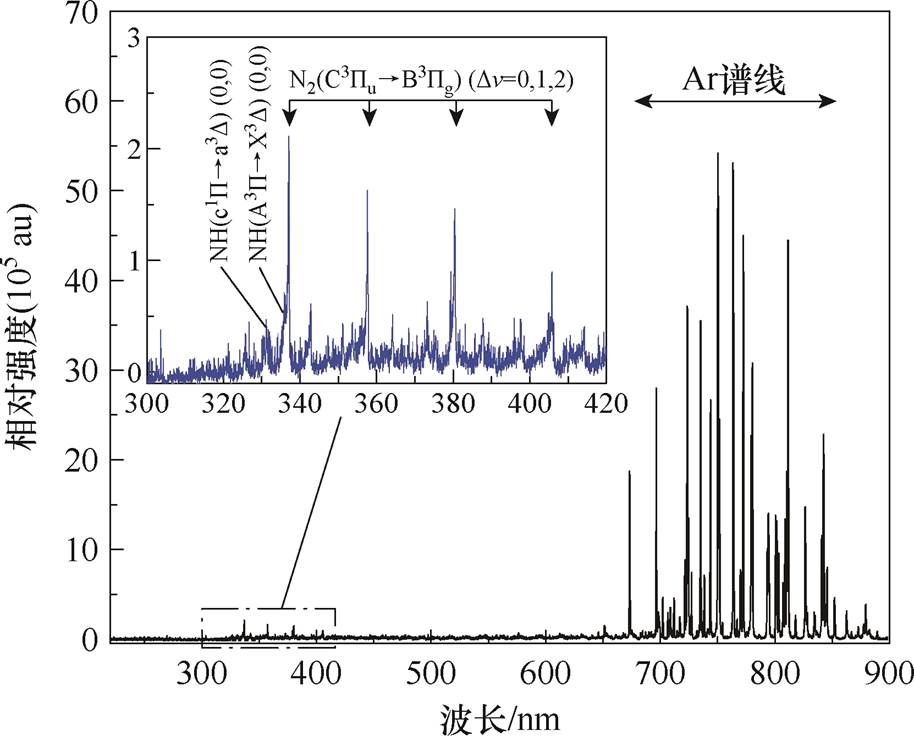
图6 实验发射光谱图像
Fig.6 Experimental emission spectrum image
结合仿真探讨了放电间隙内放电产物NH、NH2以及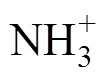 、Ar*和H的时空变化,如图7所示。从图7a~图7e可以看出,正放电阴极位降区等离子体化学反应剧烈,活性粒子密度大,
、Ar*和H的时空变化,如图7所示。从图7a~图7e可以看出,正放电阴极位降区等离子体化学反应剧烈,活性粒子密度大,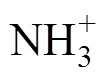 可达1018 m-3数量级。该区域中性粒子密度较大,其中NH和NH2以及亚稳态粒子Ar*峰值密度高达1018 m-3数量级。接地侧附近区域,电子与氩原子易碰撞激发形成亚稳态粒子(e+Ar→e+Ar*)[15,39-41]。而Ar*又会与NH3分子发生潘宁碰撞电离反应(NH3+Ar*→NH+ 2H+Ar,NH3+Ar*→NH2+H+Ar),产生NH、NH2,两者是生成氢气的主要中间反应物[14,42-43]。由图7e所示,H原子峰值密度高达1019 m-3数量级,集中在阴极位降区边缘附近,说明氢气主要产生于高电平时期,且在上升沿阶段之后在阴极侧介质表面附近大量积累,如图7f所示。
可达1018 m-3数量级。该区域中性粒子密度较大,其中NH和NH2以及亚稳态粒子Ar*峰值密度高达1018 m-3数量级。接地侧附近区域,电子与氩原子易碰撞激发形成亚稳态粒子(e+Ar→e+Ar*)[15,39-41]。而Ar*又会与NH3分子发生潘宁碰撞电离反应(NH3+Ar*→NH+ 2H+Ar,NH3+Ar*→NH2+H+Ar),产生NH、NH2,两者是生成氢气的主要中间反应物[14,42-43]。由图7e所示,H原子峰值密度高达1019 m-3数量级,集中在阴极位降区边缘附近,说明氢气主要产生于高电平时期,且在上升沿阶段之后在阴极侧介质表面附近大量积累,如图7f所示。
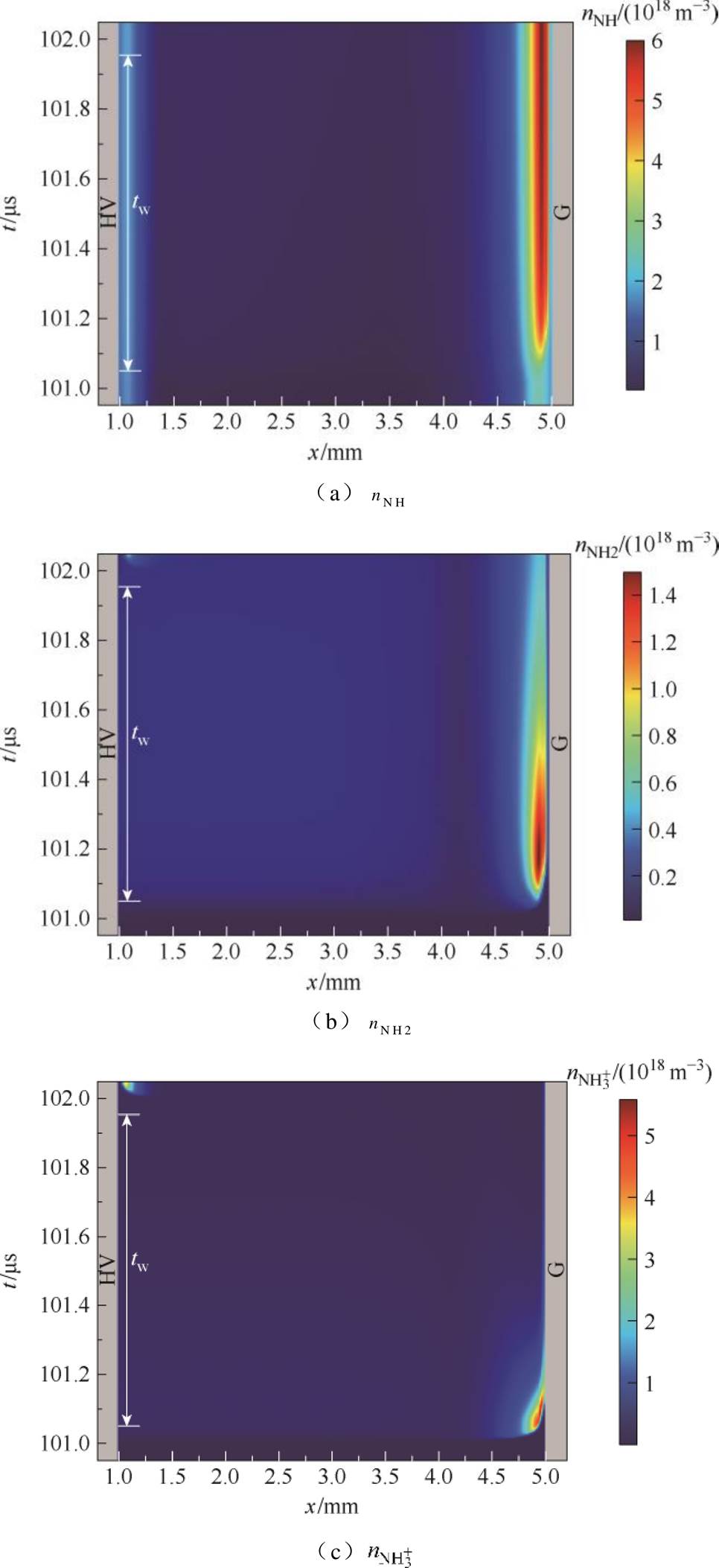
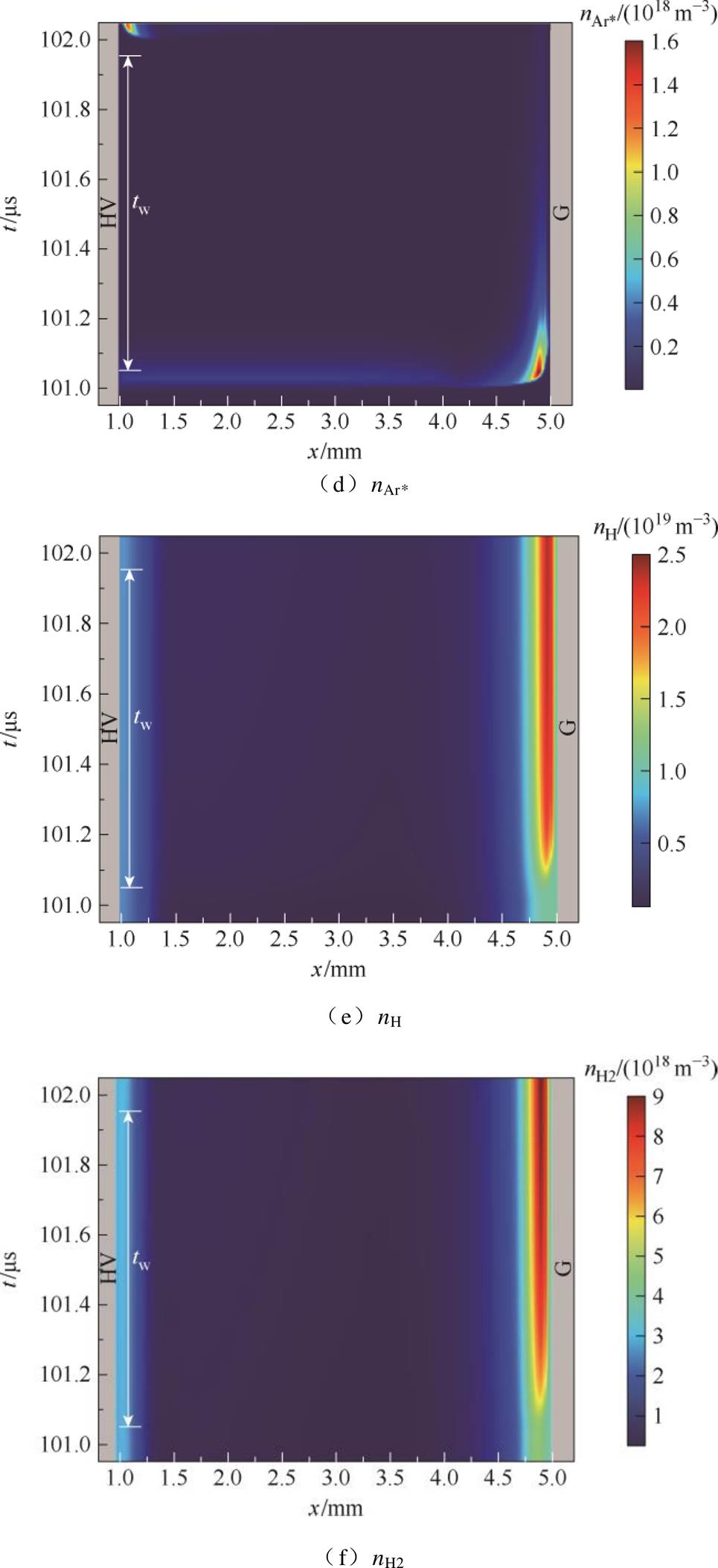
图7 一个电压脉冲期间主要粒子密度的时空分布变化
Fig.7 The spatiotemporal distribution changes of the main particle densities during a voltage pulse
为了描绘氢气的产消途径,对模型中NH3、NH2、NH、H2产生和消耗的主要反应(见表1)进行了分析,并统计了影响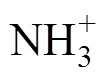 、NH、NH2、H2产消的主要反应,分别计算了脉冲电压持续时间内几种产物生成或消耗的量(通过反应速率的时间变化曲线在脉冲电压持续时间内的积分值获取),获得了主导上述产物产消反应的贡献及其占比(贡献占比是指全部相关反应中物质生成或消耗最大量占该物质生成或消耗总量的比例)。由此绘制出NH3制氢的主要路径,如图8所示。
、NH、NH2、H2产消的主要反应,分别计算了脉冲电压持续时间内几种产物生成或消耗的量(通过反应速率的时间变化曲线在脉冲电压持续时间内的积分值获取),获得了主导上述产物产消反应的贡献及其占比(贡献占比是指全部相关反应中物质生成或消耗最大量占该物质生成或消耗总量的比例)。由此绘制出NH3制氢的主要路径,如图8所示。
表1 中间产物的主要反应
Tab.1 Main reactions of intermediate products
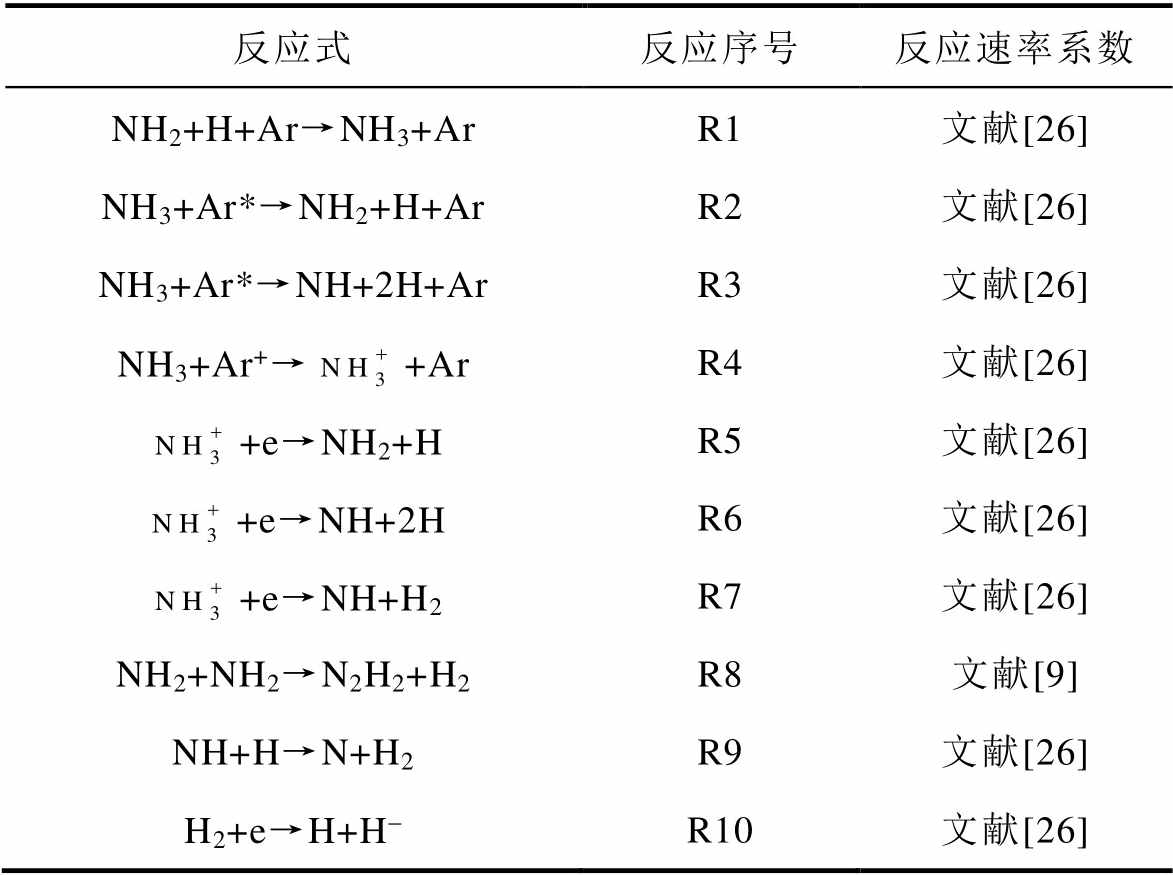
反应式反应序号反应速率系数 NH2+H+Ar→NH3+ArR1文献[26] NH3+Ar*→NH2+H+ArR2文献[26] NH3+Ar*→NH+2H+ArR3文献[26] NH3+Ar+→+ArR4文献[26] +e→NH2+HR5文献[26] +e→NH+2HR6文献[26] +e→NH+H2R7文献[26] NH2+NH2→N2H2+H2R8文献[9] NH+H→N+H2R9文献[26] H2+e→H+H-R10文献[26]
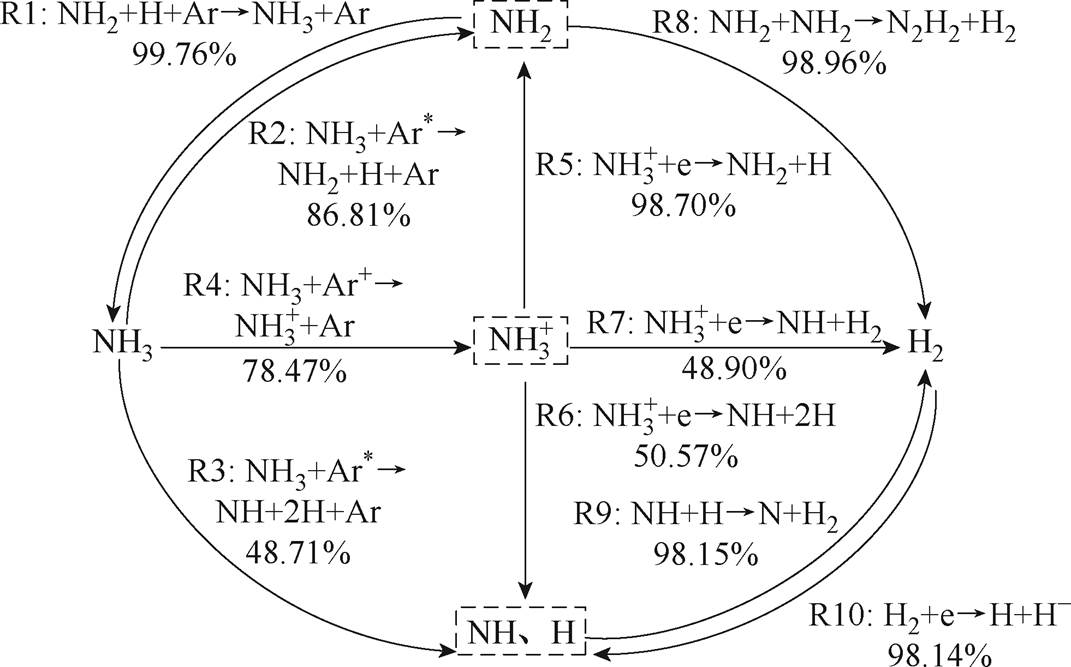
图8 NH3制氢的主要路径
Fig.8 The main path of hydrogen production from NH3
从图8可以看出,NH3主要裂解产生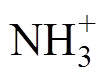 、NH、NH2等物质,反应R1为NH3的主要生成反应,在所有生成NH3的反应中占比为99.76%;其中,在NH3裂解产生NH2的所有反应中,R2反应贡献最大,占比86.81%;在NH3裂解产生NH的所有反应中,R3反应贡献最大,占比48.71%;在NH3产生
、NH、NH2等物质,反应R1为NH3的主要生成反应,在所有生成NH3的反应中占比为99.76%;其中,在NH3裂解产生NH2的所有反应中,R2反应贡献最大,占比86.81%;在NH3裂解产生NH的所有反应中,R3反应贡献最大,占比48.71%;在NH3产生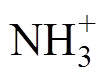 的所有反应中,R4反应贡献最大,占比78.47%。
的所有反应中,R4反应贡献最大,占比78.47%。
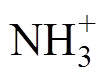 进一步复合解离产生大量NH、NH2和H等自由基,在
进一步复合解离产生大量NH、NH2和H等自由基,在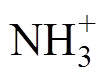 分别生成NH2、NH(H)的反应中,R5、R6的贡献最大,分别为98.70%、50.57%。而在
分别生成NH2、NH(H)的反应中,R5、R6的贡献最大,分别为98.70%、50.57%。而在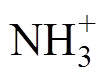 、NH2、NH作为反应物生成H2的反应中,R7、R8、R9分别对产生H2的贡献最大,R7占
、NH2、NH作为反应物生成H2的反应中,R7、R8、R9分别对产生H2的贡献最大,R7占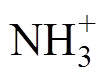 生成H2所有反应的48.90%,R8占NH2生成H2反应的98.96%,R9占NH生成H2反应的98.15%。在H2被消耗的所有反应中,R10反应贡献最大,占比98.14%。
生成H2所有反应的48.90%,R8占NH2生成H2反应的98.96%,R9占NH生成H2反应的98.15%。在H2被消耗的所有反应中,R10反应贡献最大,占比98.14%。
在本文的脉冲Ar-NH3 DBD中,间隙内NH2、NH的主导反应路径R2、R3的反应速率随时间演化如图9所示。R2和R3反应物中亚稳态Ar*的产生主要来自电子碰撞反应,因此反应速率k的峰值及变化趋势与电子密度分布相对应,其速率峰值可达18 mol/(m3·s)。随着放电过程中电子密度的增加,反应速率相应增大,正放电结束之后,电子密度逐渐减少,反应速率相应减弱。
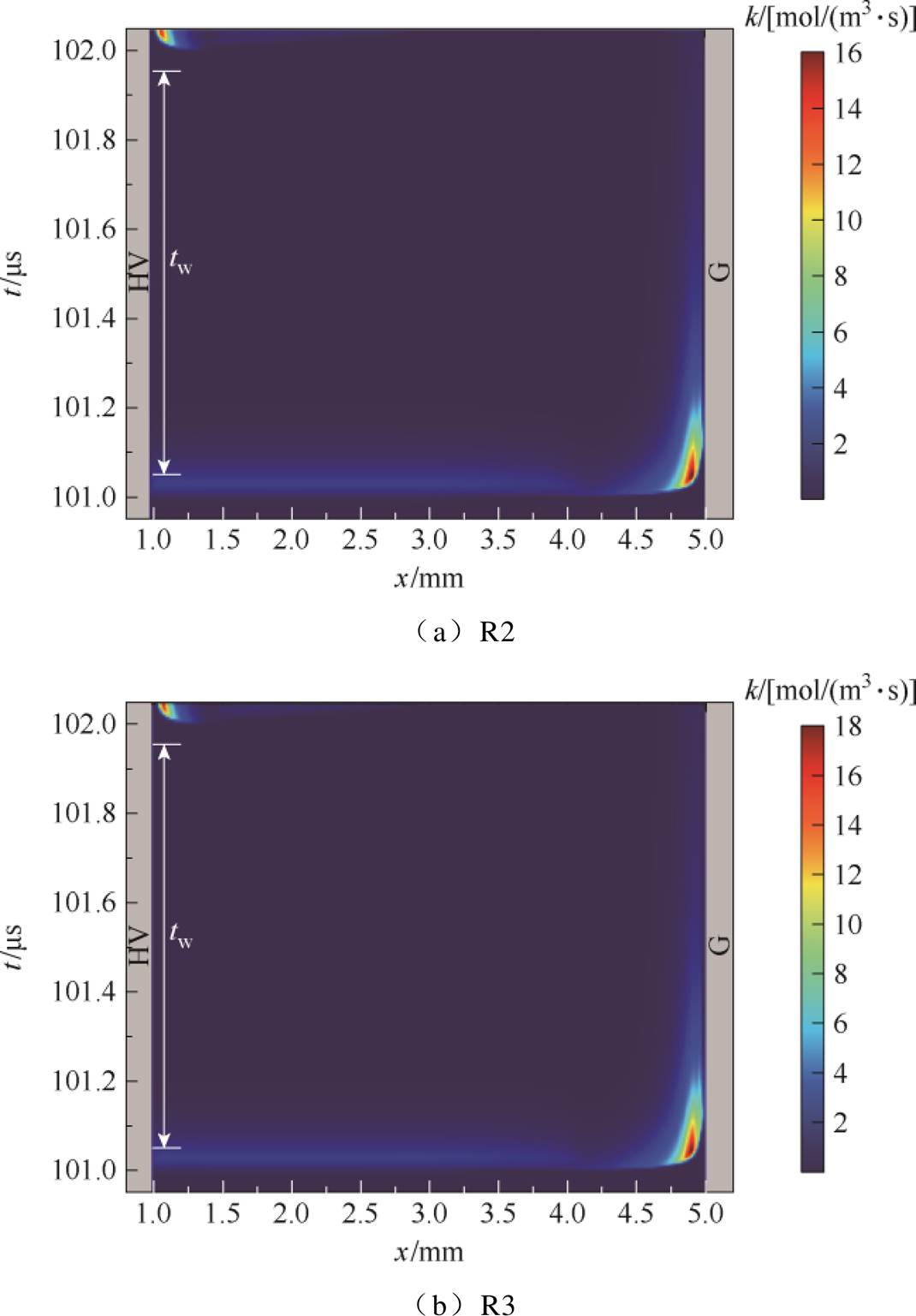
图9 一个电压脉冲期间R2、R3的反应速率
Fig.9 The reaction rates of R2 and R3 during one voltage pulse
前述H2的主要生成路径为R7、R8、R9,其反应速率如图10所示。在脉冲电压高电平阶段,接地侧阴极位降区累积了大量H2。上升沿阶段中,由于电压快速上升,一方面,初始电子在电场作用下不断获得能量,同时亚稳态氩原子碰撞激发后也将产生高能电子;另一方面,大量正粒子碰撞阴极表面产生二次电子经位降区电场加速后获得能量,接地侧位降区的电子密度越来越高,在上升沿之后达到峰值,R7反应速率与电子密度呈正相关。电子和粒子发生激发和电离反应产生大量的NH、NH2和H,并在高电平阶段累积,反应R8、R9在高电平阶段持续进行,因此,上升沿之后的高电平阶段成为生成目标产物H2的主要时期。

图10 一个电压脉冲期间R7、R8、R9的反应速率
Fig.10 The reaction rates of R7, R8 and R9 during one voltage pulse
现有研究主要集中在放电特性与机制,而对制氢效果的系统性研究较少,如M. El-Shafie等[14]通过实验研究了正弦驱动介质阻挡放电等离子体分解氨制氢系统的效率,得到等离子体膜反应器的最大产氢率为2.66%,加热或加入催化剂后最大产氢率分别可达81.6%和96.6%。基于目前的放电特性和机制研究,针对不同因素下(如驱动电压类型、反应器结构参数等)氨气(或含氨)的等离子体制氢效果研究应是未来亟须开展的主要工作,对比并优选最佳参数,为实际应用提供指导和奠定基础。
本文通过实验与数值仿真相结合,研究了纳秒脉冲电压参数对大气压Ar-NH3混合气体介质阻挡放电演化特性和产物分布的影响,得到如下结论:
1)在一个电压脉冲期间,脉冲上升沿外施电压起主导作用,气体被击穿,出现了正放电电流脉冲;而在脉冲下降沿,外施电压急剧变化,自建电场主导产生负放电电流脉冲。
2)放电呈现辉光放电特性,电子密度、约化电场强度和电子温度均在阴极位降区附近出现峰值,并在放电熄灭后的高电平起始阶段达到最大值。
3)H2主要产生于脉冲高电平阶段,并在接地侧介质表面及附近累积,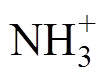 、NH和NH2是生成H2的主要中间反应物。
、NH和NH2是生成H2的主要中间反应物。
参考文献
[1] Larouci B, Bendella S, Belasri A. Numerical investigation of Ar-NH3 mixture in homogenous DBDs[J]. Plasma Science and Technology, 2018, 20(3): 035403.
[2] 梅丹华, 方志, 邵涛. 大气压低温等离子体特性与应用研究现状[J]. 中国电机工程学报, 2020, 40(4): 1339-1358, 1425.
Mei Danhua, Fang Zhi, Shao Tao. Recent progress on characteristics and applications of atmospheric pressurelow temperature plasmas[J]. Proceedings of the CSEE, 2020, 40(4): 1339-1358, 1425.
[3] Liu Jin, Zhu Xinbo, Hu Xueli, et al. Plasma-assisted ammonia synthesis in a packed-bed dielectric barrier discharge reactor: effect of argon addition[J]. Vacuum, 2022, 197: 110786.
[4] 陈星宇, 陆晨, 熊紫兰. 表面微放电等离子体特性参数计算及介质片性质的影响[J]. 电工技术学报, 2024, 39(22): 7256-7265.
Chen Xingyu, Lu Chen, Xiong Zilan. Calculation of the characteristic parameters of surface micro- discharge and the effect of dielectric sheet properties [J]. Transactions of China Electrotechnical Society, 2024, 39(22): 7256-7265.
[5] 刘定新, 张基珅, 王子丰, 等. 等离子体活化介质技术及其生物医学应用[J]. 电工技术学报, 2024, 39(12): 3855-3868.
Liu Dingxin, Zhang Jishen, Wang Zifeng, et al. Plasma-activated media technology and its biomedical applications[J]. Transactions of China Electrotech- nical Society, 2024, 39(12): 3855-3868.
[6] 张国治, 王文祥, 张磊, 等. 介质阻挡放电等离子体处理变压器废弃绝缘油的实验探究[J]. 电工技术学报, 2025, 40(1): 325-334.
Zhang Guozhi, Wang Wenxiang, Zhang Lei, et al. Experimental exploration of dielectric barrier discharge plasma treatment of transformer waste insulating oil[J]. Transactions of China Electrotechnical Society, 2025, 40(1): 325-334.
[7] 董冰冰, 孟岩, 郭志远. 气体间隙开关触发失效分析及寿命提升方法[J]. 电工技术学报, 2025, 40(1): 264-272.
Dong Bingbing, Meng Yan, Guo Zhiyuan. Trigger failure analysis and life extension methods of gas gap switch[J]. Transactions of China Electrotechnical Society, 2025, 40(1): 264-272.
[8] 陈介夫, 孙昊, 李刚, 等. 基于激光诱导等离子体的Mach-Zehnder干涉法测量气体介质恢复特性的研究[J]. 高压电器, 2024, 60(12): 199-205.
Chen Jiefu, Sun Hao, Li Gang, et al. Research on gaseous medium recovery characteristics measure- ment by Mach-Zehnder interferometry based on laser- induced plasma[J]. High Voltage Apparatus, 2024, 60(12): 199-205.
[9] Jiang Hui, Shao Tao, Zhang Cheng, et al. Comparison of AC and nanosecond-pulsed DBDs in atmospheric air[J]. IEEE Transactions on Plasma Science, 2011, 39(11): 2076-2077.
[10] 李艳琴, 张秀玲. Ar/NH3混合气体放电等离子体研究进展[J]. 真空科学与技术学报, 2017, 37(11): 1091-1101.
Li Yanqin, Zhang Xiuling. Progress of Ar/NH3mixture discharge plasma[J]. Chinese Journal of Vacuum Science and Technology, 2017, 37(11): 1091- 1101.
[11] Zhang Li, Yang Dezheng, Wang Wenchun, et al. Needle-array to plate DBD plasma using sine AC and nanosecond pulse excitations for purpose of improvingindoor air quality[J]. Scientific Reports, 2016, 6: 25242.
[12] 刘洋, 章子潇, 赵贤根, 等. 进气流量对滑动电弧放电分解CO2的瞬态电-光-热特性和转化性能的影响[J]. 电工技术学报, 2024, 39(23): 7616-7627.
Liu Yang, Zhang Zixiao, Zhao Xiangen, et al. The effect of inlet flow rate on the transient electrical- optical thermal characteristics and conversion perfor- mance of CO2 decomposition in gliding arc dis- charges[J]. Transactions of China Electrotechnical Society, 2024, 39(23): 7616-7627.
[13] 张增辉, 张冠军, 邵先军, 等. 大气压Ar/NH3介质阻挡辉光放电的仿真研究[J]. 物理学报, 2012, 61(24): 400-410.
Zhang Zenghui, Zhang Guanjun, Shao Xianjun, et al. Modelling study of dielectric barrier glow discharge in Ar/NH3 mixture at atmospheric pressure[J]. Acta Physica Sinica, 2012, 61(24): 400-410.
[14] El-Shafie M, Kambara S, Hayakawa Y. Energy and exergy analysis of hydrogen production from ammonia decomposition systems using non-thermal plasma[J]. International Journal of Hydrogen Energy, 2021, 46(57): 29361-29375.
[15] Arakoni R A, Bhoj A N, Kushner M J. H2 generation in Ar/NH3 microdischarges[J]. Journal of Physics D: Applied Physics, 2007, 40(8): 2476.
[16] Li Zhi, Zhao Zhen, Li Xuehui. Numerical analysis of a mixture of Ar/NH3 microwave plasma chemical vapor deposition reactor[J]. Journal of Applied Physics, 2012, 111(11): 113304.
[17] Niu Yulong, Li Shouzhe, Wang Xingchang, et al. Characteristic study of nitrogen microwave plasma decomposition of ammonia at atmospheric pressure for hydrogen production[J]. Plasma Sources Science and Technology, 2024, 33(10): 105018.
[18] Zhang Xinhua, Cha M S. Ammonia cracking for hydrogen production using a microwave argon plasma jet[J]. Journal of Physics D: Applied Physics, 2024, 57(6): 065203.
[19] Bazinette R, Sadeghi N, Massines F. Dual frequency DBD: influence of the amplitude and the frequency of applied voltages on glow, Townsend and radio- frequency DBDs[J]. Plasma Sources Science and Technology, 2020, 29(9): 095010.
[20] Robert R, Hagelaar G, Sadeghi N, et al. Role of excimer formation and induced photoemission on the Ar metastable kinetics in atmospheric pressure Ar- NH3 dielectric barrier discharges[J]. Plasma Sources Science and Technology, 2022, 31(6): 065010.
[21] Robert R, Massines F, Stafford L. Kinetics driving H2(a) continuum emission in low-frequency Ar-NH3 dielectric barrier discharges at atmospheric pressure [J]. Plasma Chemistry and Plasma Processing, 2024, 44(4): 1547-1561.
[22] Zhang Shuai, Wang Wenchun, Jiang Pengchao, et al. Comparison of atmospheric air plasmas excited by high-voltage nanosecond pulsed discharge and sinusoidal alternating current discharge[J]. Journal of Applied Physics, 2013, 114(16): 163301.
[23] Huang Bangdou, Takashima K, Zhu Ximing, et al. The influence of the voltage rise rate on the break- down of an atmospheric pressure helium nanosecond parallel-plate discharge[J]. Journal of Physics D: Applied Physics, 2015, 48(12): 125202.
[24] 赵勇, 王瑞雪, 章程, 等. 脉冲波形对氦等离子体射流子弹传播特性的影响[J]. 电工技术学报, 2019, 34(16): 3472-3479.
Zhao Yong, Wang Ruixue, Zhang Cheng, et al. The effect of pulse parameters on helium plasma bullet distribution properties[J]. Transactions of China Electrotechnical Society, 2019, 34(16): 3472-3479.
[25] 赵昱雷, 刘峰, 庄越, 等. 纳秒脉冲电压变化速率对氩气DBD均匀性的影响[J]. 高电压技术, 2022, 48(6): 2317-2325.
Zhao Yulei, Liu Feng, Zhuang Yue, et al. Influence of nanosecond pulse voltage slew rates on the uniformity of argon DBD[J]. High Voltage Engineering, 2022, 48(6): 2317-2325.
[26] Zhang Qian, Liu Zhiheng, Lin Deling, et al. Analysis of discharge characteristics of pulsed plasma power source in the microsecond scale[J]. Journal of Physics: Conference Series, 2022, 2313(1): 012023.
[27] Truong H T, Uesugi Y, Nguyen X B. Mechanisms of low-frequency dielectric barrier discharge (DBD) plasma driven by unipolar pulses and bipolar pulses [J]. AIP Advances, 2021, 11(2): 025022.
[28] Gong Weiwei, Huang Quanjun, Wang Zhan, et al. Effect of pulse rise time on plasma plume propagation velocity[J]. IEEE Transactions on Plasma Science, 2014, 42(10): 2868-2869.
[29] Uchida G, Takenaka K, Setsuhara Y. Effects of discharge voltage waveform on the discharge characteristics in a helium atmospheric plasma jet[J]. Journal of Applied Physics, 2015, 117(15): 153301.
[30] 赵妮, 田浩, 付强, 等. 大气压Ar-NH3混合气体氧化锆介质阻挡放电特性与产物分布[J]. 石油学报(石油加工), 2023, 39(5): 1162-1172.
Zhao Ni, Tian Hao, Fu Qiang, et al. Characteristics and product distribution of zirconia dielectric barrier discharge in Ar-NH3 mixture at atmospheric pressure [J]. Acta Petrolei Sinica (Petroleum Processing Section), 2023, 39(5): 1162-1172.
[31] Zhao Ni, Tian Hao, Fu Qiang, et al. Experimental study on the effects of gas mixture and barrier dielectric on the discharge characteristics and plasma- chemical reactions of Ar-NH3 DBD[J]. IEEE Trans- actions on Plasma Science, 2024, 52(12): 5506-5514.
[32] Zhao Ni, Yang Huan, Yao Congwei, et al. Effects of dielectric constant and secondary electron emission coefficient on discharge characteristics and products of Ar/NH3 DBD[J]. Physics of Plasmas, 2022, 29(3): 033512.
[33] Tian Hao, Zhao Ni. The effects of voltage amplitude and frequency on hydrogen by 1D fluid simulation of Ar/NH3 DBD[C]//2023 IEEE 6th International Electrical and Energy Conference (CIEEC), Hefei, China, 2023: 4120-4123.
[34] 姚聪伟, 常正实, 张冠军, 等. 大气压氩气介质阻挡放电等离子体特性变化的一维仿真[J]. 高电压技术, 2015, 41(6): 2084-2092.
Yao Congwei, Chang Zhengshi, Zhang Guanjun, et al. One-dimensional simulation of evolution characteris- ics of dielectric barrier discharge in atmospheric pressure argon[J]. High Voltage Engineering, 2015, 41(6): 2084-2092.
[35] 龙凯华, 邵涛, 严萍, 等. 高压纳秒脉冲下介质阻挡放电的仿真研究[J]. 高电压技术, 2008, 34(6): 1249-1254.
Long Kaihua, Shao Tao, Yan Ping, et al. Simulation of dielectric barrier discharges using nanosecond high voltage pulses[J]. High Voltage Engineering, 2008, 34(6): 1249-1254.
[36] Wang Weiwei, Wang Xue, Fan Zhihui, et al. Very large-cale gap distances of atmospheric pressure dielectric barrier glow discharge using semiconductor materials[J]. Europhysics Letters, 2021, 133(6): 65001.
[37] Yu Tianze, Zhang Haotian, Zhao Zhixin, et al. Characteristics of an AC rotating gliding arc discharge in NH3 and air atmospheres[J]. Physics of Plasmas, 2024, 31(2): 023501.
[38] Crispim L W S, da Silva C D, Amorim J, et al. Simulation of 1D atmospheric pressure dielectric barrier discharge in argon[J]. Physica Scripta, 2024, 99(6): 065521.
[39] Kong F R, Zhang Z L, Jiang B H. Numerical study of the discharge properties of atmospheric dielectric barrier discharge by using 200 kHz/13.56 MHz excitations[J]. AIP Advances, 2018, 8(7): 075009.
[40] Bazinette R, Paillol J, Massines F. Optical emission spectroscopy of glow, Townsend-like and radio- frequency DBDs in an Ar/NH3 mixture[J]. Plasma Sources Science and Technology, 2015, 24(5): 055021.
[41] Sun Jinguo, Bao Yupan, Ravelid J, et al. Application of emission spectroscopy in plasma-assisted NH3/air combustion using nanosecond pulsed discharge[J]. Combustion and Flame, 2024, 263: 113400.
[42] 赵越, 王丽, 张家良, 等. 非平衡等离子体放电模式及反应器结构对氨气分解制氢的影响[J]. 物理化学学报, 2014, 30(4): 738-744.
Zhao Yue, Wang Li, Zhang Jialiang, et al. Influence of non-thermal plasma discharge mode and reactor structure on ammonia decomposition to hydrogen[J]. Acta Physico-Chimica Sinica, 2014, 30(4): 738-744.
[43] Chang Zhengshi, Yao Congwei, Chen Sile, et al. Electrical and optical properties of Ar/NH3 atmos- pheric pressure plasma jet[J]. Physics of Plasmas, 2016, 23(9): 093503.
Abstract Plasma technology, characterized by low operational temperatures and broad electron energy distribution, has emerged as a promising approach for hydrogen production through ammonia decomposition. Pulse voltage has the characteristics of steep rising/falling edges and narrow pulse width. The fast voltage change rate enhances the excitation and ionization processes and has significant superiority in improving discharge uniformity and increasing particle abundance. Precise regulation of pulsed voltage-driven plasma parameters is crucial for optimizing hydrogen generation efficiency.
In this paper, under normal temperature and pressure, square wave pulse voltage is used to drive the Ar-NH3 dielectric barrier discharge (DBD) structure to generate uniform Ar-NH3 discharge plasma. The barrier dielectric is quartz with er=3.7. The diameters of the barrier dielectric are both 40 mm, the thickness is d1=d2=1 mm, and the air gap distance is dg=4 mm. The proportion of NH3 in the mixed gas is 0.5%, and the total pressure is maintained at 100 kPa. The discharge drive voltage is a pulse waveform (tr=tf =100 ns, tw=1 000 ns, f=20 kHz). The voltage and current signals, discharge evolution behavior and emission spectra of Ar-NH3 discharge plasma were tested and analyzed. To further explain the experimental results, a one-dimensional fluid simulation model was established, whose parameters were kept consistent with the experimental data. The discharge characteristics and product distribution characteristics of the Ar-NH3 DBD plasma are systematically analyzed.
The results reveal that the development process of discharge mainly includes three stages: rising edge-flat top period-falling edge. During the rising stage of the applied voltage, the air gap voltage ug increases rapidly with the increase of the applied voltage. The negative particles (mainly electrons) in the gas gap move towards the high-voltage side under the action of the electric field, and the positive ions produced by the reaction move towards the ground side. When ug reaches the gas breakdown threshold, discharge occurs, and the positive current pulse reaches its peak. The applied electric field dominates the ionization process in the gap at this time. As the discharge proceeds, negative charges (mainly ions) and positive ions continuously accumulate on the surface of the dielectrics and form stable surface charges. The self-built electric field significantly increases, resulting in a rapid decrease in the net electric field in the gap. The voltage amplitude during the pulse flat-top period remains unchanged, equivalent to a DC voltage. Due to the capacitive property of the DBD device, the gap voltage drops to zero, and the discharge remains in an extinguished state all the time. When the applied voltage pulse enters the descending stage, the applied electric field rapidly decreases. Electrons rapidly migrate to the surface of the dielectric on the grounding side and neutralize the originally accumulated positive surface charges. It rapidly weakens the positive surface charge density s2, showing a steeper curve descending edge. Meanwhile, positive ions in the gap slowly migrate to the surface of the dielectric on the high-voltage side and neutralize the accumulated negative surface charges. This causes the negative surface charge density s1 to decrease slowly, showing more gentle curve descending edge. It results in the net electric field in the gap being mainly self-built and significantly enhanced, leading to the occurrence of negative discharge and the appearance of a negative discharge peak. The discharge exhibits characteristic of glow discharge behavior with peak electron density and reduced electric field occurring near the cathode sheath region during the initial high-voltage plateau. Spectral analysis identifies dominant species including Ar* excimers, 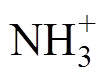 ions, and neutral NH and NH2 radicals.
ions, and neutral NH and NH2 radicals.
Reaction pathway analysis through time-integrated rate coefficients quantifies hydrogen production routes.
Primary H2 generation pathways are R7 (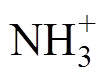 +e→NH+H2), R8 (NH2+NH2→N2H2+H2) and R9 (NH+H→N+H2).
+e→NH+H2), R8 (NH2+NH2→N2H2+H2) and R9 (NH+H→N+H2).
Mechanistic insights demonstrate that electron density accumulation during voltage rising phases originates from initial electron energy accumulation, collisional excitation via metastable Ar atoms and secondary electron emission enhanced by cathode sheath acceleration. These cumulative effects promote intensive excitation/ ionization processes, generating substantial NH, NH2 and H intermediates. Subsequent accumulation of these species during voltage plateau facilitates sustained R8 and R9 reactions, resulting in predominant H2 production near the cathode sheath region. The research results provide guidance for optimizing the hydrogen production effect and determining the optimal parameters.
keywords:Pulse dielectric barrier discharge, hydrogen generation from ammonia, discharge characteristics, discharge products
DOI: 10.19595/j.cnki.1000-6753.tces.250147
中图分类号:TM835
国家自然科学基金资助项目(52307187)。
收稿日期 2025-01-22
改稿日期 2025-04-12
赵 妮 女,1983年生,博士,讲师,硕士生导师,研究方向为复合电介质特性和等离子体催化制氢。E-mail: zhaoni@xaut.edu.cn(通信作者)
田 浩 男,2000年生,硕士研究生,研究方向为等离子体氨气制氢。E-mail: 2861273873@qq.com
(编辑 李 冰)