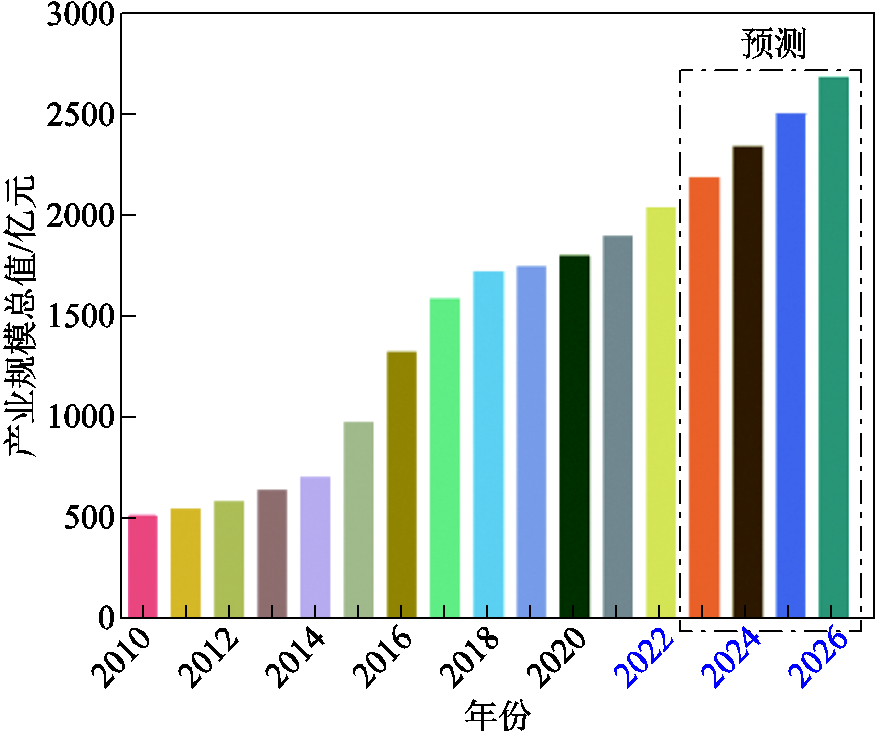
图1 全球锂离子电池产业规模情况及预测[8-9]
Fig.1 Li-ion batteries’ world market value and projected data[8-9]
摘要 锂离子电池由于能量密度高、循环寿命长、自放电率低和环境友好等优势得到了广泛应用,但其安全方面仍存在隐患,在遭遇滥用时可能会引发电池失效甚至发生火灾爆炸事故,阻碍了其在电动汽车和储能电站方面的大规模应用。针对锂离子电池的安全预警方面的研究引发了相关人员的极大关注,其中,基于电池气体分析的热失控早期预警机制相比于传统的电、热信号在可靠性、准确性、反应速度等方面有所提高。该文总结了锂离子电池在热失控过程中的气体来源,全面对比分析了触发方式、阴极材料、电池型号、荷电状态(SOC)及健康状态(SOH)对热失控产气组分、含量以及产气总量的影响规律,回顾了锂离子电池热失控过程中温度-压力演化特性的研究现状,总结了目前基于气体成分和内部压力的早期预警方案,并对现有研究的不足和潜在研究方向进行了讨论。
关键词:锂离子电池 热失控 排气行为 温度-压力演化特性 早期预警
为了应对“双碳”战略目标下的新能源革命,电化学储能技术受到了广泛的关注[1-5]。其中,锂离子电池由于比能量高、比功率高、循环寿命长、自放电率低、无记忆效应及环境友好等优点,已经广泛应用于电动汽车、大规模储能系统及便携式电子产品中[6-7]。2010—2026年,全球锂离子电池的产业规模发展趋势及预测如图1所示,预计2026年锂离子电池的全球产业规模总值能够达到2 680亿元[8-9]。然而,受组成材料的限制和应用环境的影响,锂离子电池本身具有潜在的安全隐患,也引起了相关领域的高度关注[10-12]。近年来储能电站及电动汽车火灾爆炸事故趋势如图2所示,据不完全统计,仅2021年全年就发生了10起储能电站事故和276起电动汽车燃烧事故[13-14]。由此可见,锂离子电池的安全性能提升及事故预警亟须进一步的研究。当锂离子电池遭受过充电、过放电、外部短路等电滥用,或电池副反应产热、外部环境温度过高等热滥用,或碰撞、挤压、针刺等机械滥用时,极有可能在极短的时间内发生一系列连锁放热反应,使得电池温度急剧升高,进一步引发热失控,最终导致火灾爆炸事故[15-18]。因此,为确保储能电站和电动汽车的安全稳定运行,提高其抗热失控风险的能力,研究锂离子电池的热失控早期预警技术十分必要[19-20]。

图1 全球锂离子电池产业规模情况及预测[8-9]
Fig.1 Li-ion batteries’ world market value and projected data[8-9]
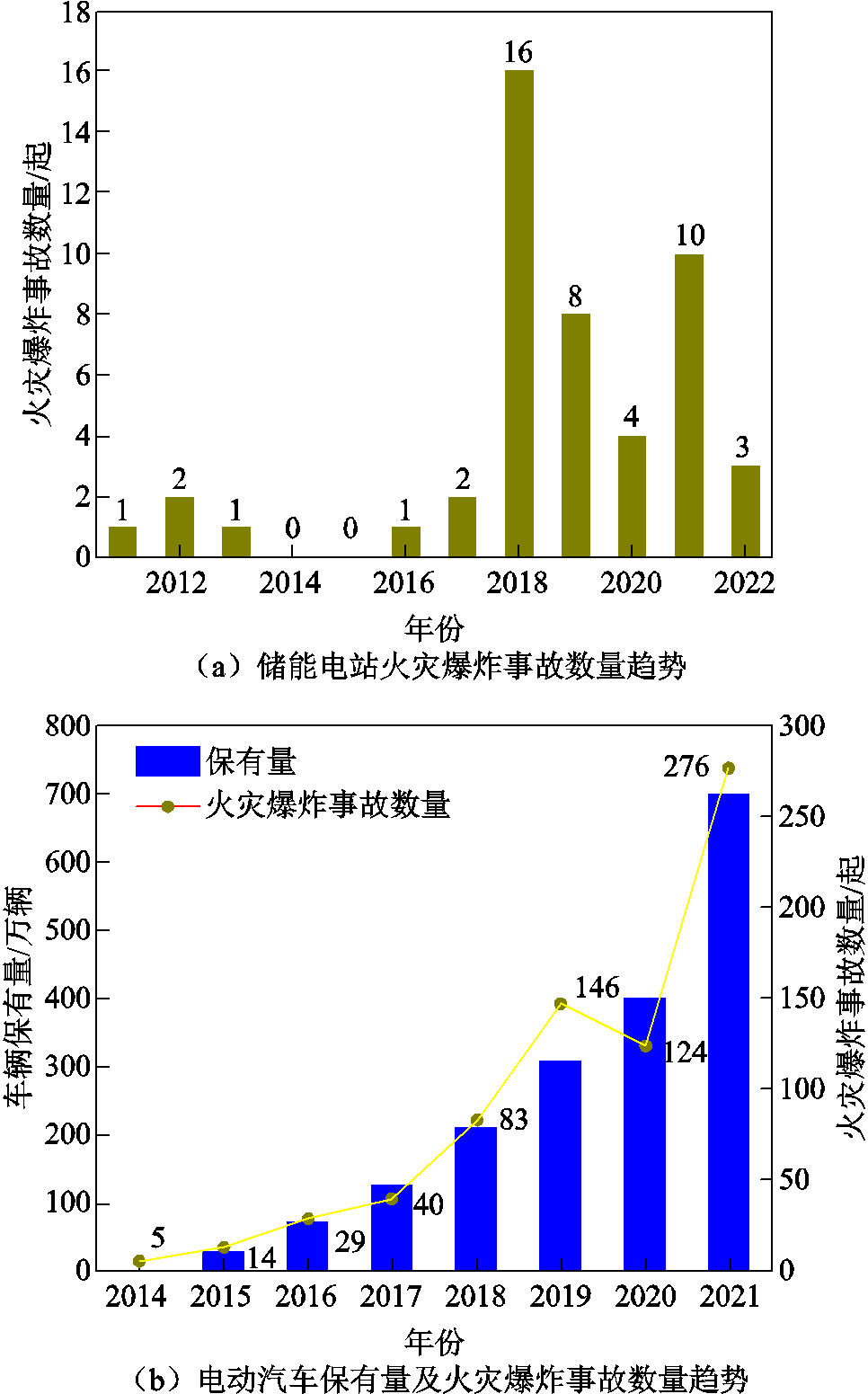
图2 储能电站和电动汽车火灾爆炸事故数量趋势图[13-14]
Fig.2 Trend of the number of accidents of energy storage power stations and electric vehicles[13-14]
基于锂离子电池热失控特征,研究人员相继提出基于电压、温度、阻抗、应变以及气体的预警方法[21-43]。Xia Bing等[21-23]提出一种基于多电压传感器的交错电压拓扑测量方案,结合智能算法、控制电路和电压阈值来监测每个串联电芯单体的端电压是否存在异常。但多电压传感器监测的缺点在于较难平衡精度和冗余度,导致成本增加。T. Grandjean等[24]通过建立数值模型来研究大倍率放电下锂离子电池的温度变化,发现电池内部和表面温度之间最大可相差20℃。后来,M. Parhizi等[25]基于热失控过程中的热导率分析,建立了LiNi0.45Co0.1Mn0.45O2(NCM)和LiMn2O4(LMO)两种阴极材料的电池内部温度跟踪模型,发现在热失控过程中,电芯内部最大温度比表面温度高接近500℃。由此可见,基于电池表面温度无法准确地监测到电池内部的真实状态。
为了进一步研究电池内部温度监测难题,L. H. J. Raijmakers等[26-27]利用电化学阻抗谱研究了电池阻抗、荷电状态(State of Charge, SOC)和温度之间的关系,将电压与电流同相位时的频率定义为截断频率f0,而阻抗的f0仅与电池内部温度有关,与SOC无关。在此基础上,R. Srinivasan等[28-29]提出一种基于70 Hz或10 Hz下电化学阻抗相移来估计锂离子电池内部温度的方法,结果表明阻抗相移与电池内部温度之间存在强相关性,而阻抗相移与电池容量之间为弱相关性;而且在热失控过程中,阻抗相位的响应早于端电压下降。但基于阻抗的方法难以实现在线预警,亟须进一步的研究[30]。A. Raghavan和A. Fortier等[31-33]开发了一种基于嵌入式光纤布拉格光栅光学传感器的锂离子电池内部状态监测装置,通过测量折射波长的变化,可以估算出电池内部的应变和温度。但光纤传感器的铺设受到锂离子电池实际使用环境和成本等方面的限制,无法做到广泛应用。在热失控早期,电池表面温度、电压等特征信号变化缓慢,电池管理系统(Battery Management System, BMS)可能无法及时监测到故障,而此时电池由于内部电化学副反应会产生大量气体[34-39],当内部压力达到一定值时会冲开安全阀,随后开始喷发[40-41]。因此,锂离子电池的排气和内压可以更为直接地反映其内部状态的变化[19]。综上所述,基于产气检测对热失控进行早期预警可能更为灵敏和准确[42]。基于电池表面温度、电压和气体技术检测热失控的预警效果对比如图3所示,可见基于气体的检测能够提前7.05 min发出警告,有效避免人员伤亡[43]。D. Sturk等也证明了气体传感器对热失控的监测要比温度传感器更加灵敏[42]。
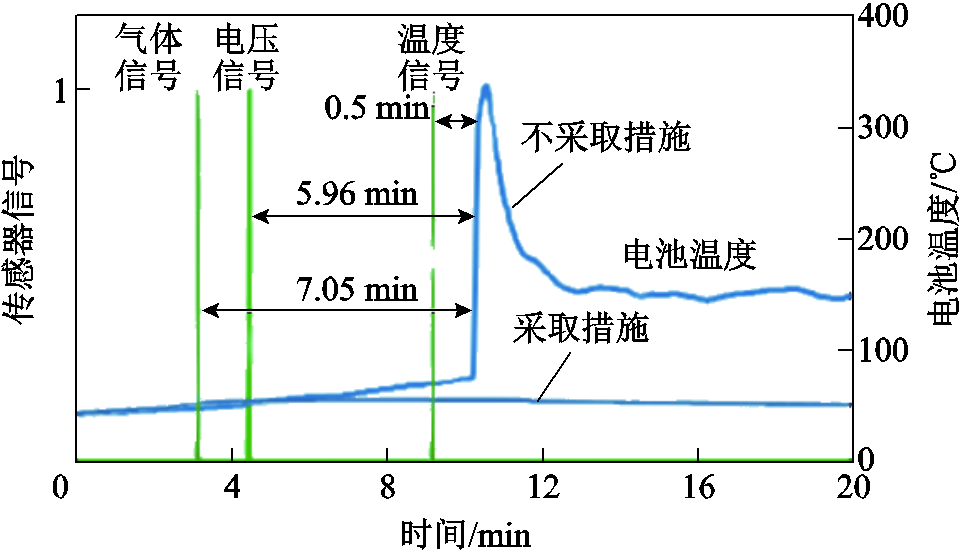
图3 基于电池表面温度、电压和气体技术检测热失控的预警效果对比[43-44]
Fig.3 Comparison of early-warning effects of battery surface temperature, voltage and gas technology for detecting thermal runaway[43-44]
基于此,本文对现有锂离子电池热失控产气来源、不同条件下的产气成分及热失控温度-压力演化特性进行总结,进一步分析基于气体的锂离子电池热失控早期预警技术,并对基于气体的热失控预警及时性和准确性的改进策略进行展望,旨在为改善锂离子电池大规模应用的安全可靠性提供指导。
当锂离子电池遭遇机械、电、热等滥用情况时,极易引发热失控,电池内部会发生一系列链式反应,迅速释放出大量热量[11]。LiCoO2(LCO)/石墨电池热失控的发展演化过程[45]如图4所示。随着温度的升高,锂离子电池内部通常经历了如下过程:固体电解质界面(Solid Electrolyte Interphase, SEI)膜分解、阳极-电解液反应、阴极-电解液反应、电解液分解反应,以及阳极-粘结剂分解反应[46]。

图4 LCO/石墨电池热失控发展演化[45]
Fig.4 The development trend of thermal runaway of LCO/graphite cell[45]
1.1.1 SEI膜分解
SEI膜是在锂离子电池首次充放电过程中,Li+与电解质溶剂、痕量水等在石墨阳极表面通过不可逆电化学反应形成的一层钝化膜。一方面,SEI膜作为“保护层”,允许锂离子通过,并且防止溶剂组分和电子通过,能够避免电解液继续还原分解导致充电状态下电极受到腐蚀;另一方面,SEI膜可能会使部分石墨阳极活性材料失活[47]。SEI膜主要由稳态组分(如Li2CO3和LiF)和亚稳态组分(如ROCO2Li、(CH2OCO2Li)2、ROLi和含氧聚合物)组成[48]。SEI膜一般在90~120℃时开始发生分解反应,反应路径[49]为
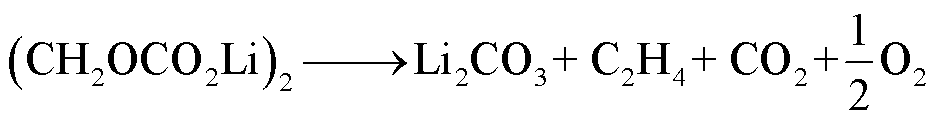 (1)
(1)
此外,阳极嵌锂可能与SEI膜发生反应生成Li2CO3和C2H4[50],或者与CO2发生还原反应生成CO[47],反应路径[51-52]分别为
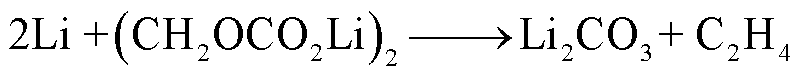 (2)
(2)
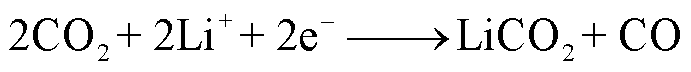 (3)
(3)
SEI膜中的亚稳态组分也可能会与痕量水或者HF(来源于电解液分解)反应生成CO2,其反应路径[38,47,53]分别为
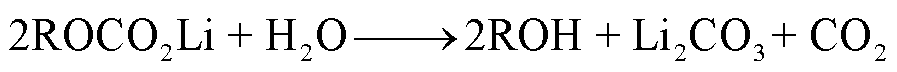 (4)
(4)
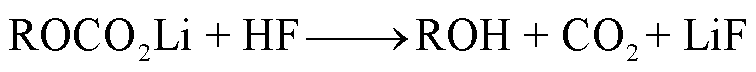 (5)
(5)
高温会造成SEI膜的降解和破裂,一旦SEI膜在高温下分解,暴露出的石墨阳极会再次发生电化学反应生成SEI膜[34]。在120~250℃之间,SEI膜重分解-再生反应同时发生[34]。
1.1.2 阳极-电解液反应
由于SEI膜的分解,阳极失去保护作用而暴露于电解液中,此时电解液与石墨阳极之间也会发生化学反应[54]。Wang Qingsong等[55]利用C80微型量热仪对嵌锂石墨阳极和电解液的热行为进行了研究,单独的LixC6暴露于高温下只有一个放热峰,对应SEI膜分解;而在LixC6-1.0 mol/L LiPF6/碳酸乙烯酯(Ethylene Carbonate, EC)+碳酸二乙酯(Diethyl Carbonate, DEC)电解质体系中观察到四个放热峰,分别对应SEI膜分解、Li-电解质反应、SEI膜再生与再生SEI膜分解以及Li-聚偏氟乙烯(Polyvinylidene Fluoride, PVDF)的反应。对于不同的x碳阳极(x为不同数量的插层锂),放热反应的起始温度范围为51~69℃[55]。当阳极嵌入锂的量越多,化学反应的活化能越低,反应产热量越多[55]。阳极插层锂与电解液反应会释放出易燃的碳氢化合物,可能的降解反应路径[55-56]为
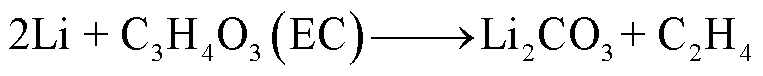 (6)
(6)
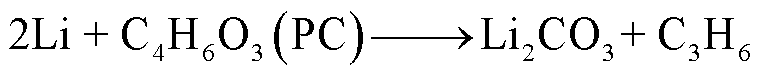 (7)
(7)
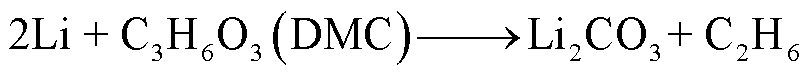 (8)
(8)
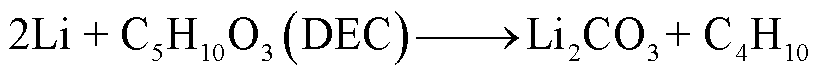 (9)
(9)
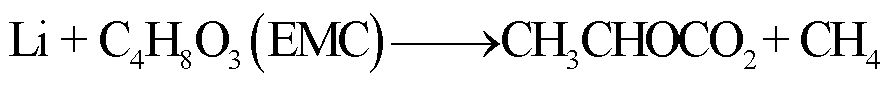 (10)
(10)
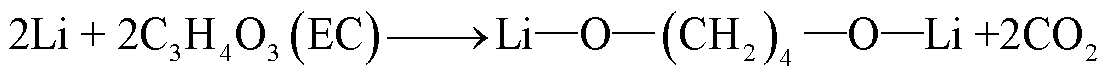 (11)
(11)
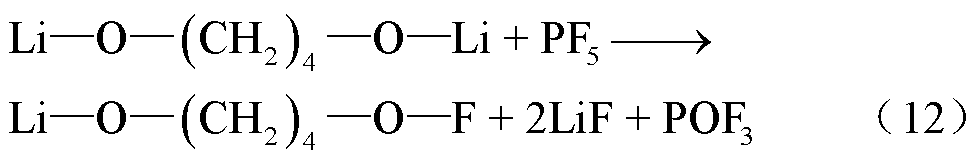
阳极材料和电解液的易吸水性、电池注液和存放环境都可能导致电池内部存在痕量水,阳极嵌入锂可以和水分发生反应,从而产生H2,反应路径[57]为
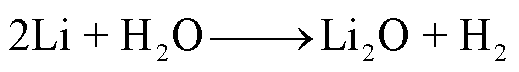 (13)
(13)
阳极石墨或者炭黑(导电添加剂)可能发生燃烧,生成CO,反应路径[57]为
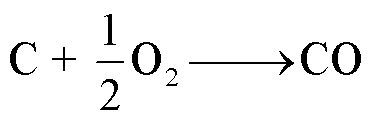 (14)
(14)
1.1.3 阴极分解及阴极-电解液反应
随着温度升高,阴极材料开始发生放热分解反应并释放氧气[58]。一般来说,氧气与电解液的放热副反应是造成热失控的主要原因(因为放热较多),而释氧量取决于阴极的锂化状态[57]。由于实际情况下电池充放电过程中存在不可逆的容量损失,阴极不能通过电池放电完全锂化,即使在SOC为0%的情况下,阴极也可能会释放少量氧气[57]。D. H. Doughty和E. P. Roth总结了不同阴极材料的热稳定性,从高到低[59]为:LiFePO4(LFP)>LMO>NCM>Lix(Ni0.8Co0.15Al0.05)O2(NCA)>LCO。
1)LCO
LCO电池热稳定性相对较差,一般在220~500℃之间发生分解反应[60],分解路径[61-63]为
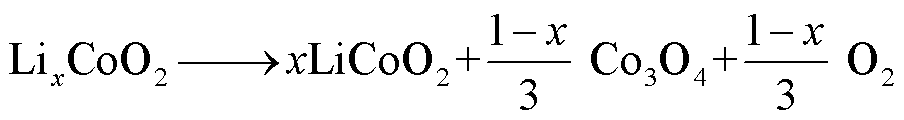 (15)
(15)
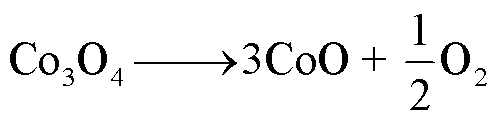 (16)
(16)
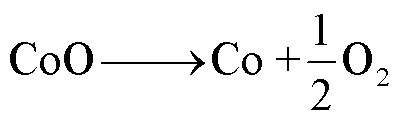 (17)
(17)
2)NCA
在175~600℃之间,锂化的NCA阴极材料经受相变(从层状结构过渡到岩盐结构)而释放氧气,反应路径[57]为
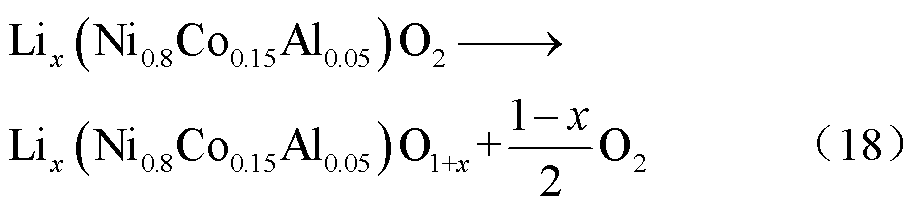
3)NCM
NCM阴极材料的热分解强度低于LCO和NCA,且其性能取决于氧化物中Ni、Co和Mn原子的比例[64]。一般来说,Ni含量越高、Co和Mn含量越少,则NCM容量越高,但相变起始温度越低,且释氧量越大[65-67]。NCM分解反应路径[64]为
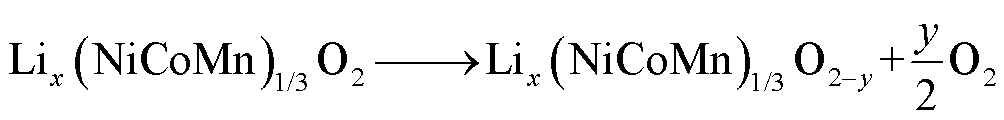 (19)
(19)
4)LMO
LMO阴极材料的热稳定性随着电解质浓度的增加而降低[68],Z. Zhang等[69]和P. Biensan等[60]利用差示扫描量热仪(Differential Scanning Calorimetry,DSC)实验研究了锂离子电池组分的稳定性,结果分别发现LixMn2O4-电解质混合物在225~400℃和150~300℃之间发生放热反应,分解反应路径[60,68-69]为
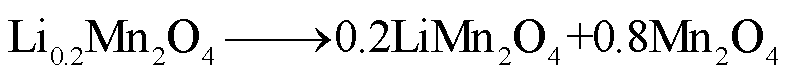 (20)
(20)
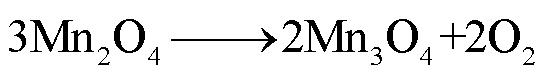 (21)
(21)
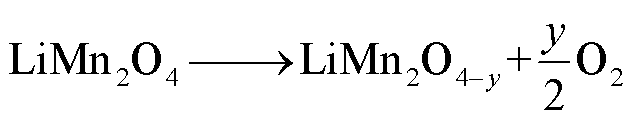 (22)
(22)
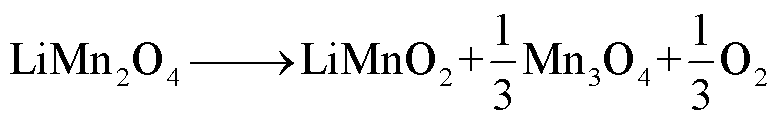 (23)
(23)
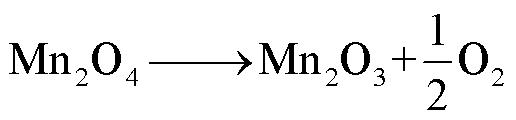 (24)
(24)
5)LFP
与LCO、NCM阴极材料相比,LFP阴极材料具有相对更好的热稳定性,在310℃时才发生分解[70-71],这是因为LFP中磷酸基团(PO4)3-八面体结构的强P=O共价键[72]。P. Röder等[73]和J. Kim等[74]通过X射线衍射(X-Rays Diffraction, XRD)观测到FePO4在受热过程中发生了释氧的相变,假设锂离子电池中的LFP阴极由锂化和非锂化粒子的混合物组成,则LFP释放氧气的路径为
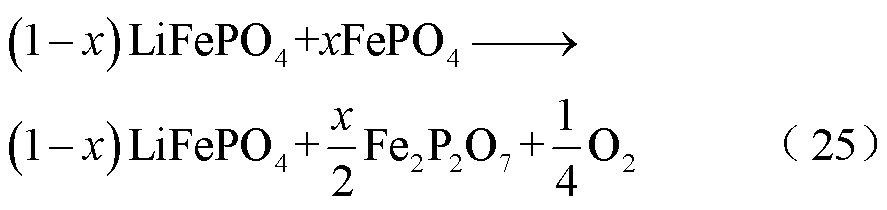
1.1.4 电解液分解反应
电解液一般由碳酸二甲酯(Dimethyl Carbonate, DMC)、EC、碳酸丙烯酯(Propylene Carbonate, PC)等有机溶剂和锂盐LiF6组成,电解液反应一般包括锂盐的分解反应及其产物与溶剂的反应、溶剂的氧化反应以及痕量水的电解反应[75]。当温度超过200℃时,有机溶剂将会发生放热分解反应并释放出CO2、CO、氟化物和碳氢化合物[76-77]。而且,环状溶剂(EC、PC等)的热稳定性通常比链状溶剂(DMC、DEC、碳酸甲乙酯(Ethyl Methyl Carbonate, EMC)等)更高,链状溶剂在共存体系中的反应活性排序[55]为DMC>EMC>DEC。
在高温下,锂盐LiF6遇热发生分解或水解,其产物包括强Lewis酸的高活性物质PF5、HF、POF3等有毒物质[78-79]。PF5和POF3作为活性中间体,存在时间较短,可以与其他有机物质或水发生反应,最终生成HF[36]。根据美国的保护行动标准(Protective Action Criteria, PAC),气态HF具有超强的毒性和高腐蚀性,在体积分数为24×10-4%下具有不可逆且严重的健康影响,而在体积分数为44× 10-4%时可能会威胁生命安全[80-81]。此外,从其氯的类似物POCl3/HCl来看,POF3的毒性甚至可能比HF的毒性更大[36]。LiF6的反应路径[82-84]为
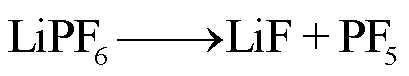 (26)
(26)
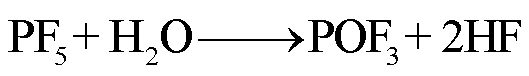 (27)
(27)
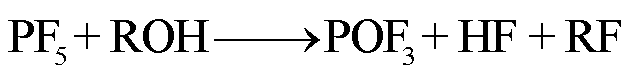 (28)
(28)
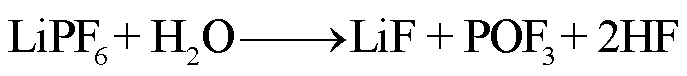 (29)
(29)
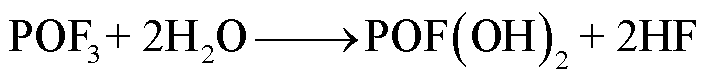 (30)
(30)
随着温度升高,电解液会与阴极释放的O2发生反应,当O2有限时,一些碳氢化合物只能还原成CO,反应路径[85-86]为
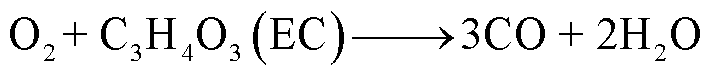 (31)
(31)
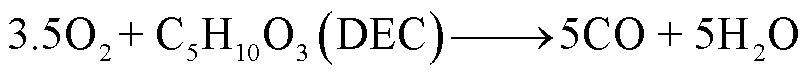 (32)
(32)
 (33)
(33)
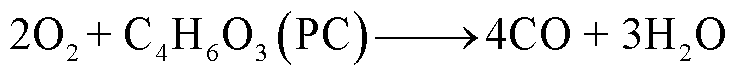 (34)
(34)
当O2含量相对充足时,上述产物主要为CO2和H2O[87-88]。
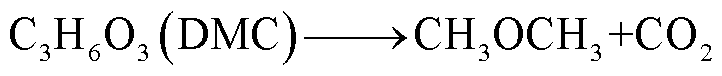
此外,锂盐分解产物将会使得有机溶剂的热稳定性降低,发生进一步的分解反应[89-93],反应路径为
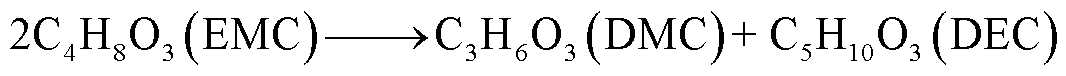 (39)
(39)
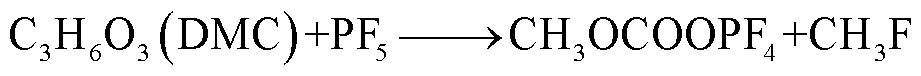 (40)
(40)
 (41)
(41)
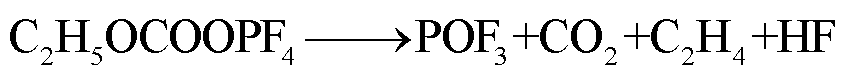 (42)
(42)
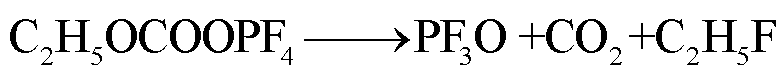 (43)
(43)
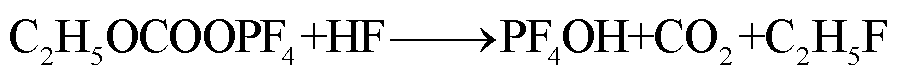 (44)
(44)
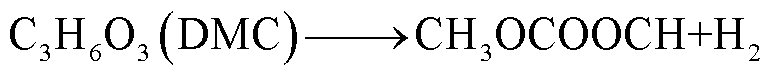 (45)
(45)
 (46)
(46)
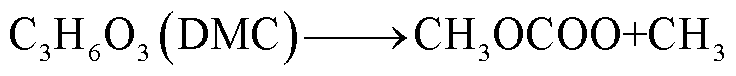 (47)
(47)
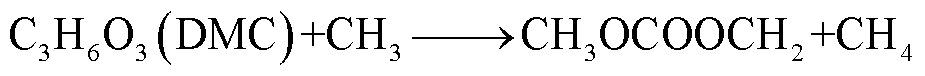 (48)
(48)
1.1.5 阳极-粘结剂分解反应
在温度高于230℃时,阳极石墨颗粒脱落,锂直接暴露在周围的电解质和粘结剂中[94];当温度超过260℃时,粘结剂(如PVDF)可能会与阳极材料或者LixC6反应生成H2和氟化物等气体[10, 57]。然而,PVDF粘结剂在电解液中分解反应的化学动力学对全电池的热失控行为影响较小,在热失控反应中无法与锂溶剂竞争[95-96]。
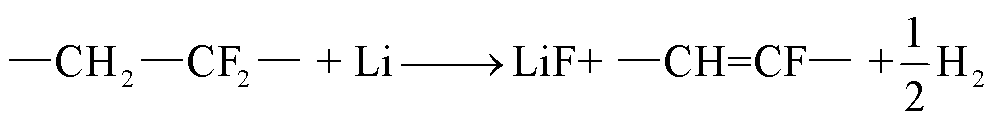 (49)
(49)
随着上述锂离子电池内部化学反应的发生和气体分解产物的积累,锂离子电池内部的压力上升,从而触发安全阀的开启[40,49],释放出的可燃气体将会进一步增加与热失控相关的危险。
分析锂离子电池在热失控过程中的危害和产气行为具有十分重要的意义。首先,有助于更加准确、深入地理解热失控机理;其次,可以为利用气敏技术早期检测不同条件下的热失控提供理论基础。根据现有研究,分析锂离子电池产气行为和特性的手段主要有三种:①微尺度的热测试,如热重分析(Thermal Gravimetric Analyzer, TGA)DSC联用气相色谱/质谱仪(Gas Chromatography/Mass Spectrometry, GC/MS),或者傅里叶变换红外光谱仪(Fourier Transform Infrared Spectrometer, FT-IR Spectrometer)[56,84,97];②开放空间下锂离子电池在正常循环、外部加热、过充或其他滥用条件下释放气体,基于气体分析仪在线监测产气的组分和含量[38,98-101];③利用密封容器收集废气并传输到GC/MS或FT-IR,进行产气组分和占比的分析[10,37,85,102-104]。前两种方式为原位监测,第三种为非原位测试。材料体系、触发条件、SOC、健康状态(State of Health, SOH)、环境条件(含氧量是否充足)甚至检测手段都会对产气结果造成影响。基于前人实时监测气体的研究成果,电池热失控前主要释放电解液挥发气体,一般也会检测到小部分的CO、H2、C2H4、CH3OCHO、HF和CH3OCH3[37]。此外,单体电芯和成组电池的热失控产气组分没有明显区别[34, 105]。
1.2.1 不同热失控触发方式下的产气成分分析
通常情况下,热失控测试的要求为具有与现场的相关性、真实性、可重复性、可操作性等[106],针刺和过充触发热失控的实验一般缺乏可重复性。通过对比事故中的滥用情况,过充和过热诱导下的热失控要比其他滥用下的反应更加剧烈和严重,这是因为热量和电量的输入导致锂离子电池内部能量增加[11,107]。但不同触发方式下的热失控主要产气成分相似,产气中各气体的摩尔分数如图5a所示,Y. Fernandes等[37]发现LFP电池在过充至热失控过程中的产气成分与热滥用后[57]的相同,但产气总量差异较大,他们将此归因于过充和过热下电池内部的温度梯度不同,即过充过程中电池内部存在热点,而热滥用测试中电池被均匀加热[37],温度分布较为均匀。热滥用下的产气危险性要高于针刺引发的排气,例如,F. Diaz等[35]研究了锂离子电池热失控排出的有毒气体,结果表明,针刺穿透引发热失控产生的有毒气体总量和质量损失都明显少于热滥用下的情况,他们也总结了不同因素对锂离子电池排气毒性的影响,如图5b和图5c所示。此外,K. Kumai等[39]比较了商用18650 LCO电池在过充和过放下的产气情况,抽取了充放电后电池内部的气体进行检测,发现两种循环条件下的主要产物均为CO2和碳氢化合物,这是因为电解液发生了化学分解反应。而且,由于在过放电电池中检测到了微量CO和O2,推测过放电后阴极材料发生了释氧相变。此外,过放电产气总量几乎为过充电的4倍[39]。因此,过放电循环中产气量更大、气体毒性危害也更大。
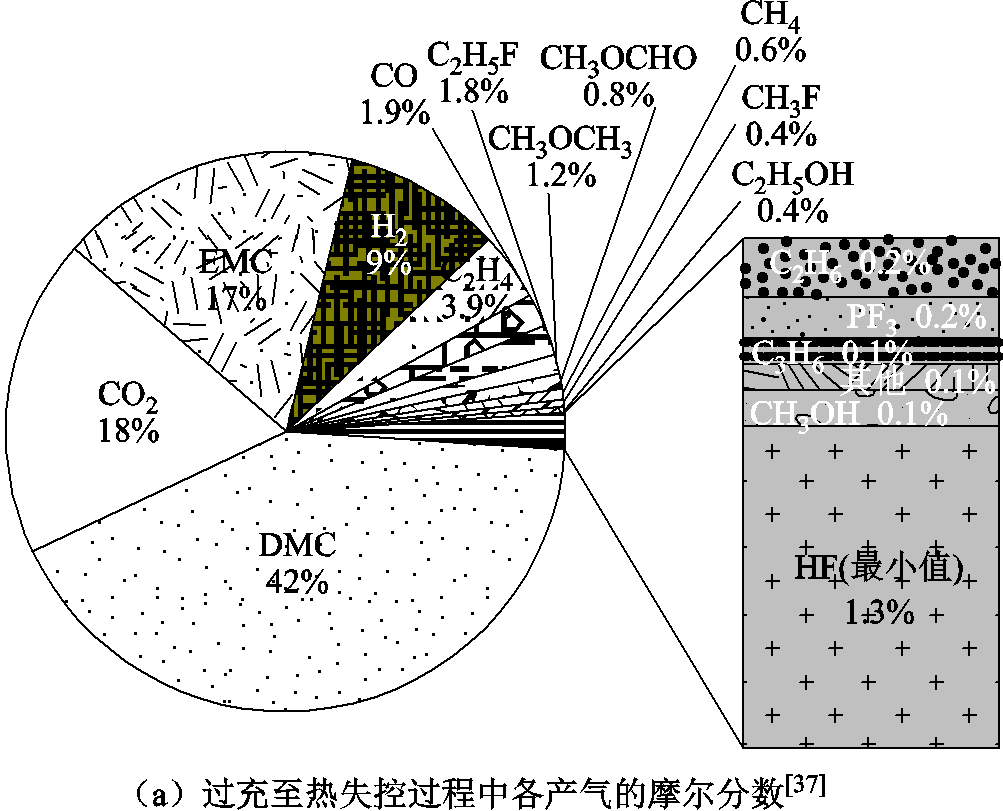
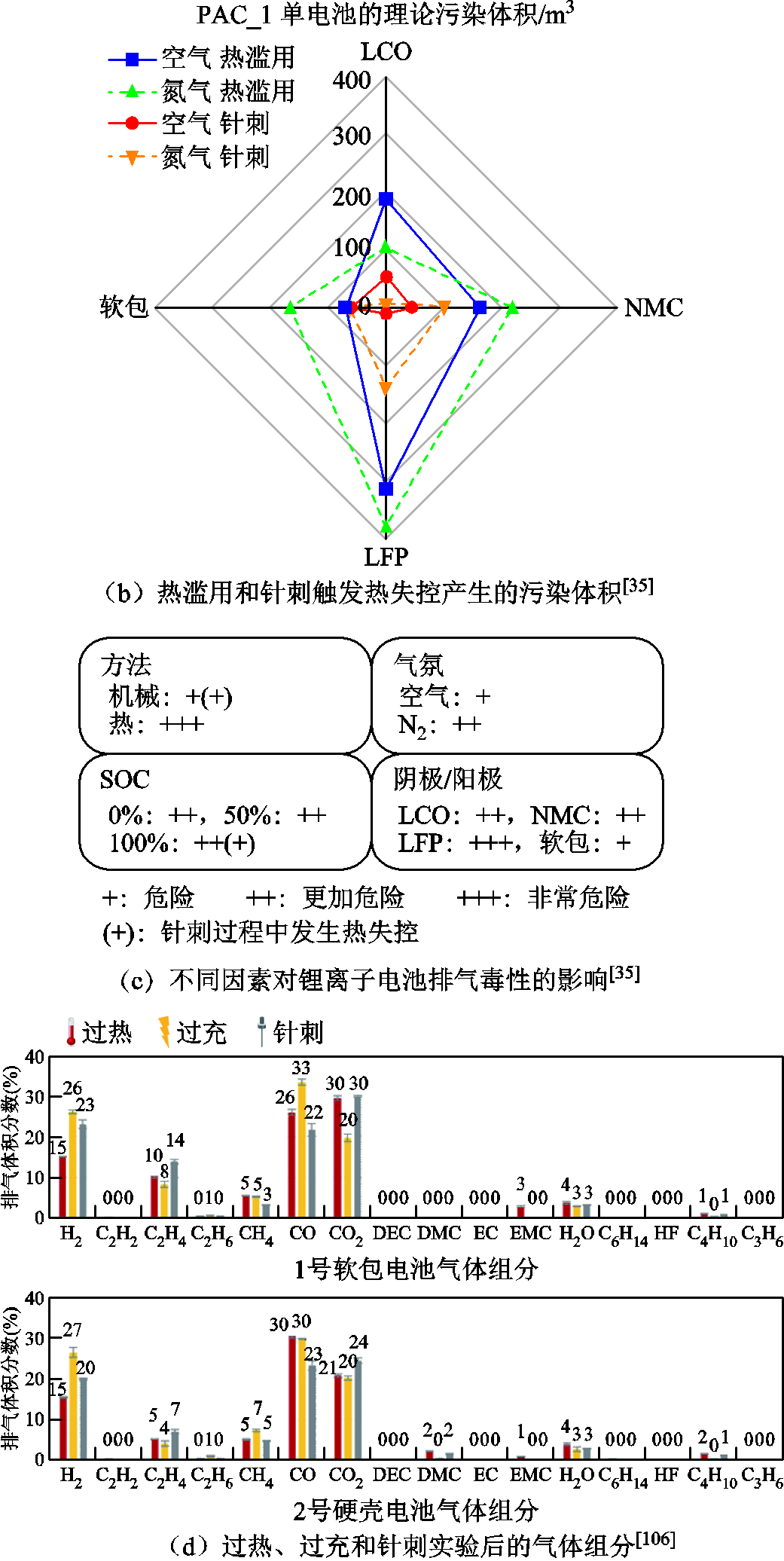
图5 不同触发方式下的产气行为[35,37,106]
Fig.5 The gas production behavior under different triggering methods[35,37,106]
不同触发方式下的排气行为也有所不同,即会影响排气次数、排气速率、排气总量和产物含量等。C. Essl等[106]分别采用了过热、过充和针刺穿透触发60 A·h NCM电池的热失控,以研究其热行为和排气行为,实验后的气体组分如图5d所示。结果发现,在过热和过充实验中均出现了两次排气,其中一次在热失控开始之前,而针刺实验只在热失控开始后发生一次排气事件,主要的排气产物均为CO2、CO、H2、碳氢化合物和电解质溶剂。而且,不同触发方式下排气中电解质含量的变化非常明显,其中过充触发下排气中不包含电解质溶剂挥发气体,说明过充下电池内部温度增加较快,电解液溶剂的化学分解反应发生得较为彻底[106]。此外,在过充实验中,排气总量、排气速率和质量损失都较大,过充、过热和针刺条件下的产气总量分别为2.8 L/(A·h)、 1.6 L/ (A·h)和1.7 L/(A·h),排气速率为过充>针刺>过热,而且过充情况下易燃易爆和有毒气体(CO、H2、CH4等)的体积分数要高于过热和针刺实验,这是因为过充后电池能量增加、阴极严重脱锂变得不稳定、阳极表面出现金属锂沉积,再次印证了过充引发的热失控要比针刺和过热诱发的更加严重[11]。
1.2.2 不同阴极材料下的产气成分分析
不同阴极材料释氧能力有所不同,进而会影响电池的排气行为和燃烧特性[16],如图6a所示。Kong Weihe等[38]比较了LCO、LMO和LFP圆柱形自组装电池在正常循环和过充状态下的气体种类,发现在正常充放电状态下,气体组分与阴极材料无关,仅仅是总量有所不同,主要气体包括CO2、C2H6、C2H4、CH4和C2H5F。而在过充条件下,C2H2气体的含量不同,且与阴极材料的释氧能力有一定的相关性:即氧化能力较弱,将会产生更多的C2H2;氧化能力较强,则产生更多的CO2。因此,认为C2H2可作为比较阴极材料氧化能力的探针,且三种阴极材料在过充状态下的氧化能力依次为LCO>LMO>LFP,这一结论与Chen Mingyi等[108]的相吻合。A. O. Said等[109]将LCO、NCM和LFP阴极材料的18650电池成组排列,每组测试中电池数量都多于100节,结果表明,电池组热失控产气组分仍为CO、CO2、H2和碳氢化合物,LFP电池的碳氢化合物、CO和CO2的质量产率大约比LCO和NCM电池低一个数量级。此外,在LCO和NCM电池组的产气中检测到了少量O2,而在LFP电池组中则未检测到,进一步说明了LFP阴极材料的释氧能力较低。
不同阴极材料的锂离子电池释放出的气体总量和产物含量不同。A. W. Golubkov等[57,85]比较了LCO/ NCM、NCM、LFP和NCA商用18650锂离子电池的热失控产气特性,部分结果如图6b所示,发现在100% SOC下,产气的主要成分均为CO2、CO、H2及少量的碳氢化合物。此外,产气中CO的质量分数排序为NCA>LCO/NCM>NCM>LFP。考虑到CO非常容易引起人体窒息,NCA、LCO/NCM和NCM电池的产气毒性比LFP电池更大。结合LCO/NCM、NCM、LFP、NCA电池产气总量分别为265、149、50、273 mmol,因此,热失控产气危害性由高到低为NCA>LCO/NCM>NCM>LFP。由于A. W. Golubkov等的检测技术受限,并没有检测出HF、POF3等氟化物。

图6 不同阴极材料的电池的产气行为[16,35,85]
Fig.6 The gas production behavior of cells with different cathode materials[16,35,85]
阴极材料对电池热失控产生的氟化物有毒气体的影响很大[34,36,42]。D. Sturk等[42]发现LFP电池的排气速度慢且废气总量较少,产气中HF的含量高于NCM/LMO电池,但两种电池产气中HF的总释放量相近。F. Diaz等[35]发现LFP电池热失控释放气体比LCO和NCM电池更为危险,由HF、COF2、丙烯醛、CO、HCl、甲醛和电解质溶剂挥发气体引起的理论污染量占LFP排气总量的96%以上,如图6c所示。相比于HF气体,关于POF3的滥用研究很少,两者的产量比通常在8:1和53:1之间[110]。F. Larsson等[36]定量研究了LCO、LFP和LiNiCoAlO2-LiAlTiPO4(NCA-LATP)几款商用电池在燃烧时排放出的有毒气体HF和POF3,只有0% SOC下的LCO电池能够监测到POF3的存在,且其归一化总产量为15~22 mg/(W·h);此外,几款电池的HF产量不尽相同,最高的为20 A·h LFP软包电池,产量达到150~198 mg/(W·h),最低的为3.2 A·h圆柱LFP电池和6.8 A·h硬壳LCO电池,分别为12~24 mg/(W·h)和15~25 mg/(W·h)。
1.2.3 不同型号电池的产气成分分析
不同类型(软包、圆柱、方形硬壳等)电池的热失控过程中的“基本效应”是相似的,即会出现电池电压降落、自产热、气体产生、电池破裂及粒子喷射等现象[105]。S. Koch等[111]对51种NCM大容量锂离子电池进行了热诱导失控实验,探究了电池容量、气体氛围对热失控产气组分的影响,发现热失控产气组分不受产气量的影响,大容量电池热失控产气组分与小型电池基本一致[57,85]。然而,电池及其排气设计会影响电池的热失控行为,进而导致电池的热响应、排气量和排气速度的差异[35,112]。硬壳电池通常有一个安全阀,作为外壳中压力承受最薄弱的区域,安全阀在电池内部压力达到规定值时打开,避免发生爆炸事故。通常情况下,硬壳电池可以比软包电池承受更高的内部压力[113]。F. Larsson等[100]发现方形硬壳电池在热失控过程中出现三个峰,分别对应三次排气周期。而P. Ribière等[99]对软包电池进行量热测试,结果显示只有一个峰与电池热失控相对应,这是因为软包电池缺乏排气阀,初始时通过焊缝失效而排气,这种排气发生在内部压力较低的情况,且释放气体量较少,在量热实验中很难观测到。Huang Lüwei等[114]对40 A·hNCM软包和方形电池进行了1C(将电池在1 h完全放电的电流速率)过充失效实验,软包电池的表面最高温度高于方形电池,但前者具有更好的耐过充能力。此外,电池的设计、样式和包装材料对热传播也会造成一定的影响。根据F. Larsson等[36]对于不同型号LFP电池的产气研究,软包电池相比圆柱和方形电池会释放出更多HF气体,原因可能是硬壳方形电池和圆柱电池在爆裂前会产生更高的压力,迅速从电解质中释放出大量的气体或蒸汽,由于释放速度快、反应时间短,使得燃烧反应不完全,最终导致反应产物较少。此外,M. Lammer等[115]对三种型号的18650 NCA电池进行了热滥用实验,分析了电池在第一次排气、热失控和燃爆三个阶段的产气组分和含量,发现在燃爆过程中检测到的气体产量最高,其中INR18650—35E型号的电池产气量高达224.6 mmol,ICR18650—32A型号的电池CO较高,为58.41%。这表明尽管锂离子电池的阴极材料和额定容量是相似的,但其热失控产气含量差别可能较大。
1.2.4 不同SOC下的产气成分分析
SOC对锂离子电池热失控的产气总量和产气行为具有显著的影响,因为电池内部能量会随着SOC的增加而增加,所以热失控的严重程度也将随之加剧[116-118]。热失控的产气总量通常与SOC呈正相关[57,119-123],例如,Jiang Fengwei等[120]将18650 LCO电池加热到热失控,随着SOC的增加,LixC6中会有更多锂与溶剂反应生成可燃气体,导致热失控产气总量增加。而且,不同SOC下阴极、阳极与电解质的反应如图7a所示,可见,随着阴极脱嵌程度的增加会释放出更多O2[121],阳极上的沉积锂将会与O2发生剧烈的化学反应[122],引起更大的内压,导致内部材料可能会喷射出来并形成火焰[123]。A. W. Golubkov等[57]和V. Somandepalli等[119]也报道了相似的结论,随着SOC的增加,热失控产气总量大幅增加,产气中CO2含量明显降低,H2和CO的趋势则相反,说明热失控气体成分含量与SOC相关。Y. Fernandes等[37]基于FT-IR实时监测了过充-热失控过程中26650 LFP电池的排气行为,过充过程中释放出的气体含量趋势如图8所示,即:当电池过充至SOC为120%时,内部压力达到外壳破裂点,释放的主要气体成分及含量排序为DMC>CO2>CO>EMC CH4;放热反应持续进行,电池内部温度逐渐积累,由于隔膜熔断电池电流降为零,SOC约为135%,产气中逐渐出现CH3OCH3、H2、CH3OCHO和C2H4等气体;此后除继续释放上述可燃气体之外,HF开始出现且含量持续增加,推测是空气中的痕量水渗透到电池内部与剩余的LiPF6发生反应所生成的。热失控结束后发现,主要产气中DMC、CO2、EMC和H2占产气总量的42%、18%、17%和9%,还存在微量HF和PF3等有毒气体,推测是因为实验过程中电池温度较低,导致产气中含有大量的电解液蒸汽[37]。
CH4;放热反应持续进行,电池内部温度逐渐积累,由于隔膜熔断电池电流降为零,SOC约为135%,产气中逐渐出现CH3OCH3、H2、CH3OCHO和C2H4等气体;此后除继续释放上述可燃气体之外,HF开始出现且含量持续增加,推测是空气中的痕量水渗透到电池内部与剩余的LiPF6发生反应所生成的。热失控结束后发现,主要产气中DMC、CO2、EMC和H2占产气总量的42%、18%、17%和9%,还存在微量HF和PF3等有毒气体,推测是因为实验过程中电池温度较低,导致产气中含有大量的电解液蒸汽[37]。
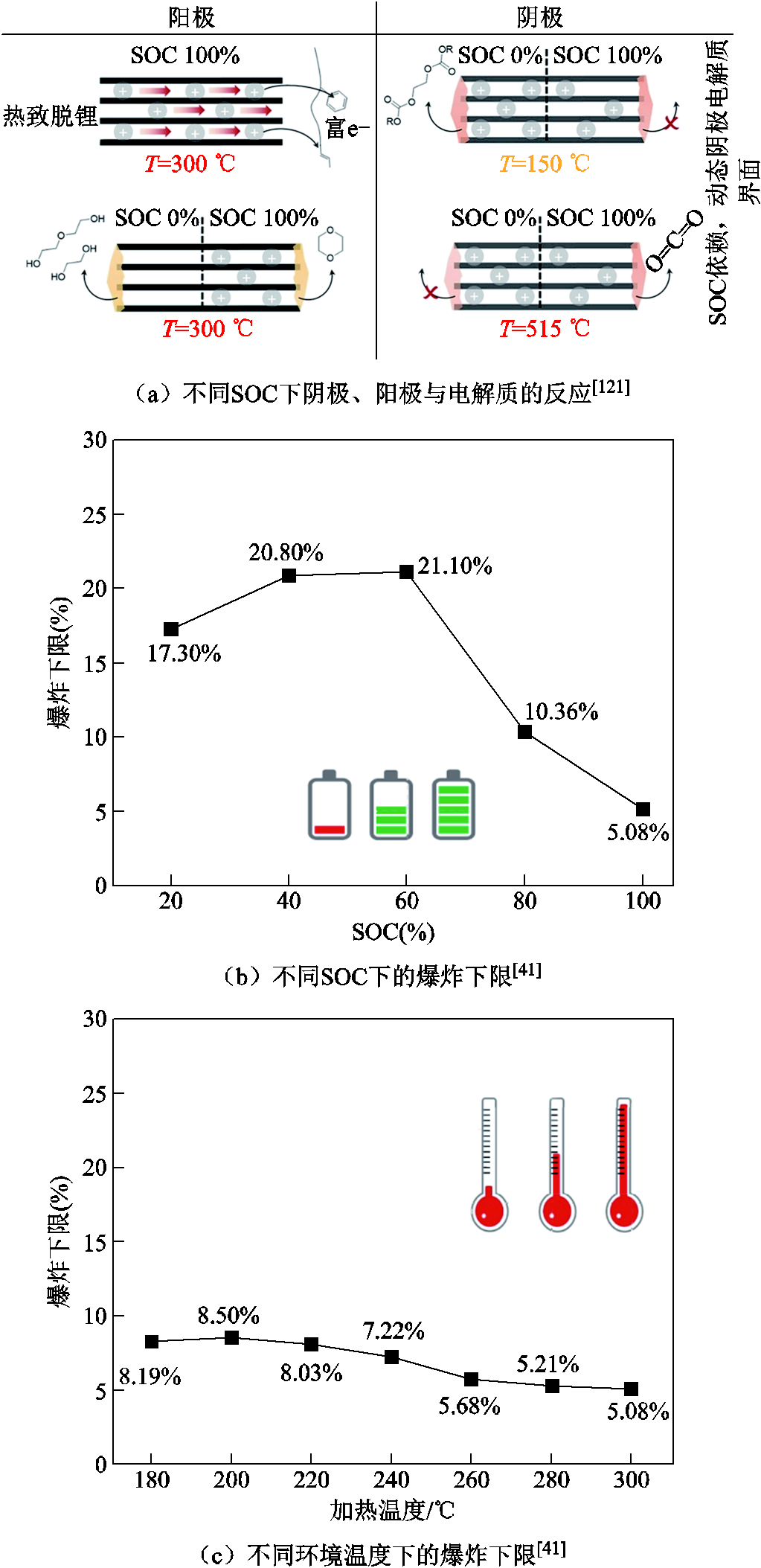
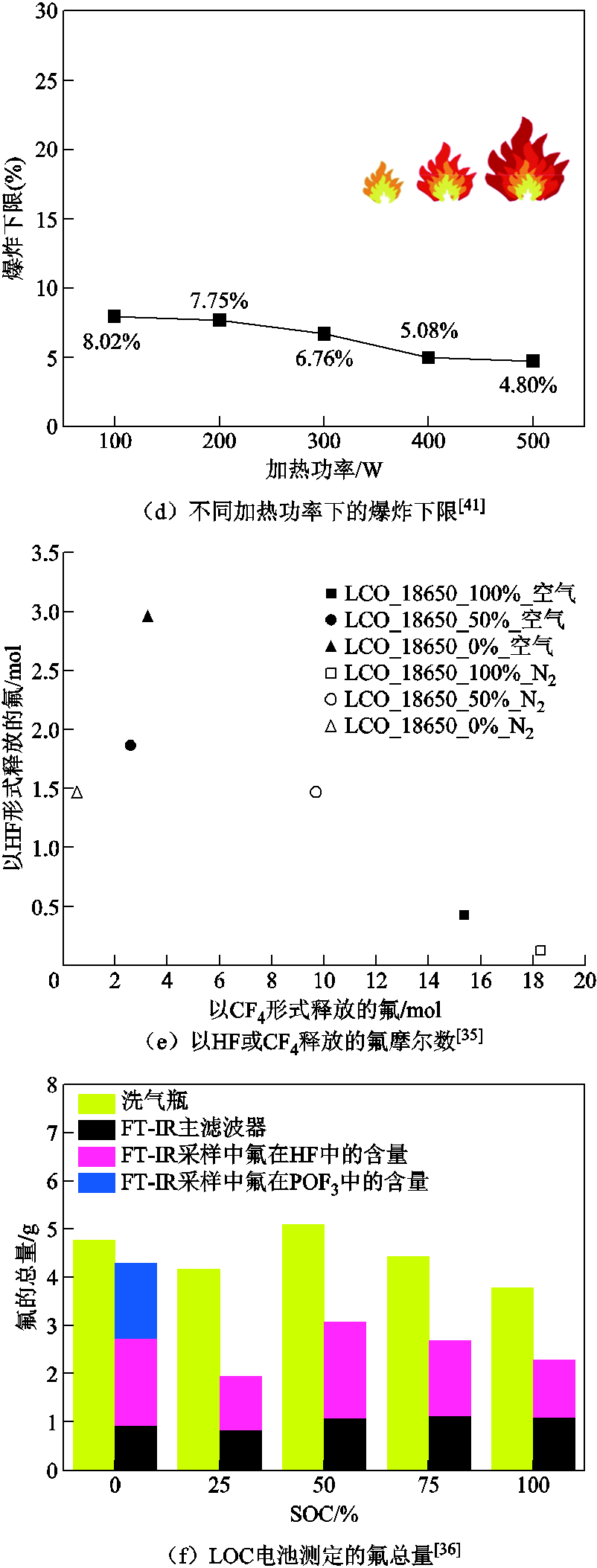
图7 不同SOC下的产气行为[35-36,41,121]
Fig.7 Gas production behavior under different SOCs[35-36,41,121]
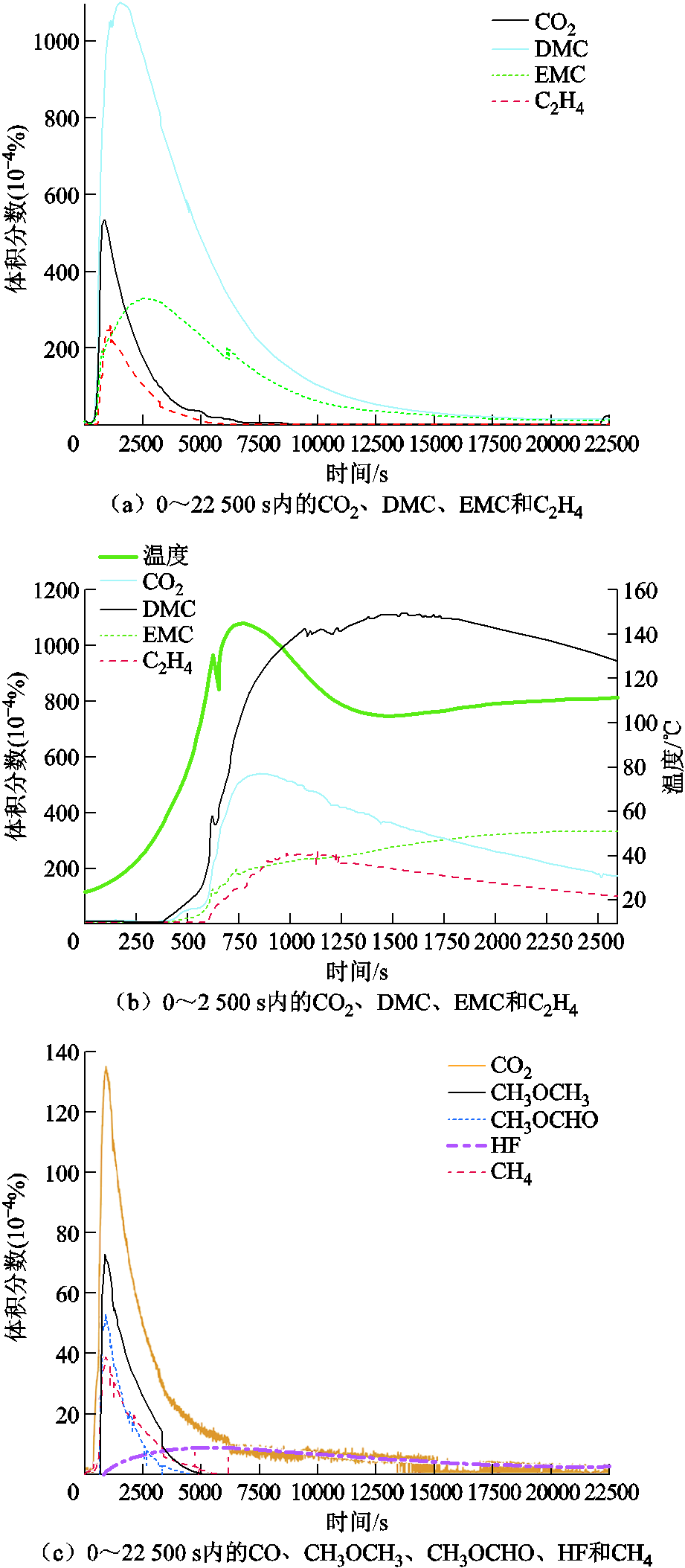
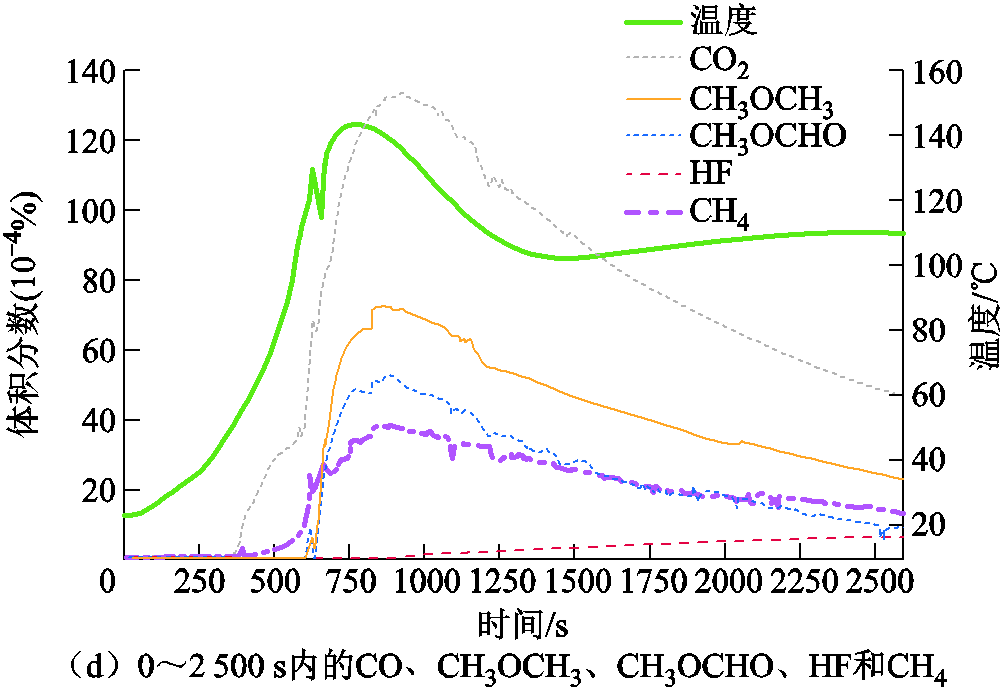
图8 过充过程中释放的气体浓度与时间的关系[37]
Fig.8 Relationship between gas concentration released during overcharge and time[37]
此外,不同SOC下的热失控气体种类数量以及爆炸极限也有所差异[40,116,124]。Zhang Qingsong等[124]基于实验并结合经验公式,研究了18650 LCO电池的热失控气体组分和爆炸极限。结果表明,热失控气体种类随着SOC的升高而增加,例如,在0% SOC下热失控排气中仅检测出CO2、CO、C3H6O3和C4H8O3四种气体,但在100% SOC下检测出了31种气体成分。随着SOC的增加,爆炸上限的变化趋势与不饱和烃含量的变化趋势相同,先减小后增大;而爆炸下限的变化趋势与烷烃含量的变化趋势一致,先升高后降低,在SOC为50%下表现出最低的燃爆危险[124]。Liao Zhenghai等[118]针对NCM电池进行热滥用实验也发现了相似的规律,在5%、50%、90%和100% SOC下分别识别出6、10、15和25种气体成分。此外,Chen Shichen等[41]基于GC-MS和FRTA爆炸极限仪研究了不同SOC、环境温度和加热功率下的热失控产气组分和爆炸下限,如图7b~图7d所示,CO和CH4的含量会随着环境温度和SOC的增加而增加,爆炸下限也随着环境温度和外部加热功率的增加而降低;而随着SOC的增加,爆炸下限先增大后减小,在100% SOC条件下爆炸极限最低,热失控危害较高。
SOC也会影响锂离子电池热失控过程中排出气体的毒性[35-36,99,125-126]。Sun Jie等[125]研究了SOC对有毒燃烧产物的影响,不同SOC下识别出的燃烧有机物的种类数量见表1,发现在100% SOC下的产气会导致最严重的毒性。而且,随着电池容量的增加,产物中CO的含量迅速上升,在容量为2、3、6.5、10、30 A·h时,CO含量分别为616×10-4%、1 100×10-4%、3 900×10-4%、14 000×10-4%和45 500×10-4%,而其余有毒气体含量变化却不明显,即萘、苯、戊二烯、苯乙烯、甲苯、二甲苯和茚的含量仍保持在几十10-4%的水平。然而,如图7e和图7f所示,F. Diaz等[35]发现SOC对热滥用下的LCO电池产生的产气毒性没有明显影响,但气体类型随SOC改变而发生明显变化,即HF的总量随着SOC的增加而降低,但CF4逐渐增加。原因是HF在低SOC下更加稳定,其含量在完全放电状态下达到最大值,而在高SOC下形成了未被检测到的氟化物CF4,这一结论与P. Ribière[99]、A. Lecocq[126]和F. Larsson等[36]的结论相吻合。对于这种现象,J. Scheirs等[127]给出了初步解释,即更高的温度和更高的加热速率会加剧键断裂和小分子的产生,但没有给出形成CF4的具体机制。此外,硫基化合物一般作为添加剂用于电解质中,有助于SEI膜的形成。在高温下它们会降解形成SO2,而SO2的含量会随着SOC的增加而增加[128-129]。HCl的形成来源于粘结剂、隔膜和包装中的聚合物,且HCl的产量与SOC无关[130]。
表1 不同SOC下的锂离子电池燃烧排出的气体种类数量[125]
Tab.1 Types of gases emitted from the combustion of lithium-ion batteries at different SOCs[125]

阴极材料0% SOC50% SOC100% SOC150% SOC LCO627356 LMO723309 NCM8142518 LFP15103
1.2.5 不同SOH下的产气成分分析
保证锂离子电池在整个使用寿命期间的高安全性至关重要,锂离子电池的使用情况复杂多变,导致其老化过程也是非线性且复杂的[131-133],如存储期间的高环境温度、高SOC通常会使得锂离子电池衰减加快[134-138];使用期间的高低温环境、大倍率放电、脉冲放电、不同放电深度(Depth of Discharge, DOD)、不同SOC区间使用,甚至过充电、过放电等滥用[139-141],也会促使锂离子电池的性能削弱,可能发生如沉积锂、电极分层、活性材料损失和锂离子损失等现象影响电池的形态和完整性[142-143]。老化电池的热安全性取决于老化的途径和所考虑的度量,从热力学方面来看,老化后的电池电化学容量衰减,导致其相比于新电池在热失控过程中具有更低的峰值温度和总放热量,因而老化电池通常更为安全[144];但从动力学方面来看,老化后的锂离子电池自热反应和热失控起始温度降低[139,141],发生反应程度更加强烈的热失控,从而降低了电池的安全性[140,143]。通常情况下,锂离子电池在经受日历或循环老化后的热失控过程中,释放的气体总量会降低,而可燃气体占比将会增加[145],且产气成分也取决于老化历史[146]。而且,由于老化循环过程中内部气态电解质降解产物的积累,热失控过程中内部电流中断装置(Current Interruption Device, CID)激活时间可能更早[147]。
老化锂离子电池的排气行为也会随之改变[100,141]。例如,A. Friesen等[141]报道了热失控过程中新电池会随着SOC的增加产生更多的气体产物,而老化电池的放热反应则不存在SOC依赖性。F. Larsson等[100]对商用6.8 A·h的锂离子电池进行了不同程度的老化后触发热失控,研究其排气行为,结果如图9所示,发现老化后的电池均出现了三次独立的排气事件,其中前两次在热失控发生之前,释放出DMC和乙酸乙酯(Ethyl Acetate, EA)蒸汽,而第三次与热失控同时发生,安全阀完全打开,释放出大量的烟雾和气体,除DMC和EA外还释放出CO、EC、HF和POF3(图上未显示)。从图9中可以看出,循环100圈和300圈的测试结果相似,但循环100圈的电池排气发生时间更早,排放气体总量更多,可见在电池循环到100~200圈之间时存在一个局部的最小值,更易引发电池热失控。
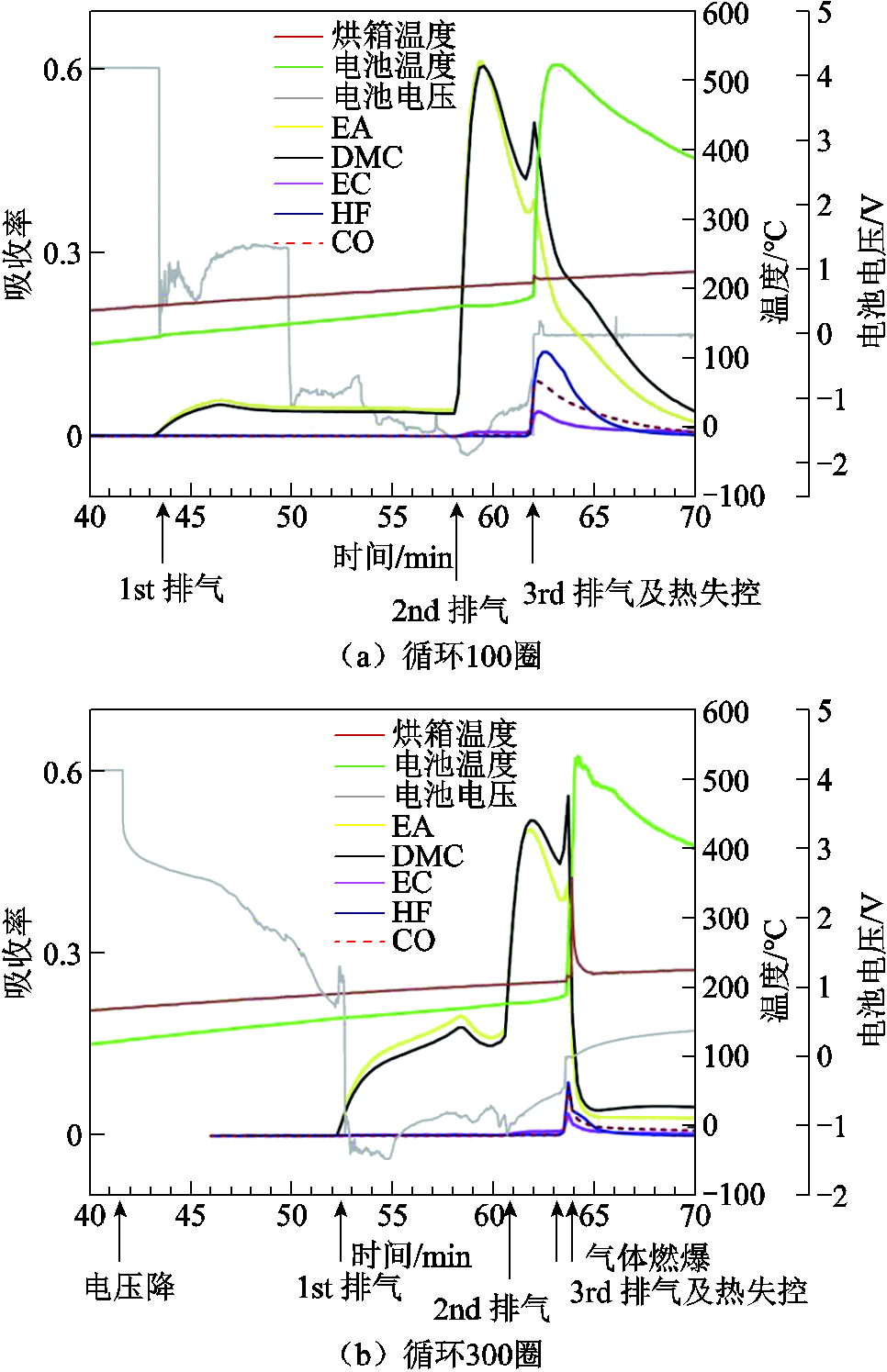
图9 循环不同圈后热失控过程中的电池电压、温度和气体排放[100]
Fig.9 Cell voltage, temperature and gas emissions during thermal runaway after various cycles[100]
综上所述,热失控触发条件,以及锂离子电池的组成材料、SOC、SOH等不同时都会对产气结果造成影响,典型文献中不同因素影响下的锂离子电池产气分析结果见表2。
此前的研究已经分析了电解液和单体电芯级别的热分解气体含量和组成,但是关于排气现象的物理本质或排气对热失控温度演化的影响的研究也至关重要[148-149]。已经有研究证明,基于气压的热失控预警要早于基于电池表面温度的预警[102],甚至可能早于基于产气组分的识别[150]。因此,研究锂离子电池产气压力的演化过程十分重要。
基于现有研究,目前关于锂电池内部气压的研究方法主要有三种:①基于密封压力罐开展热失控实验,通过测量罐内的压力变化,推测电池热失控的产气速率,以研究其喷发过程[119,151-157];②基于引压管的电池内部压力测试,在对电池造成有限影响的情况下在电池负极打孔并引入引压管,进而实现测量电池的内部压力[158-159];③结合化学反应动力学、计算流体力学等方法,建立数学模型以模拟产气过程,从定量角度揭示热失控过程中特征气体的释放特征[148-149,160-163]。
锂离子电池在热失控过程中可能产生10.3 MPa甚至更高的压力[164],例如,T. Y. Lu[151]和T. H. Dubaniewicz[165]分别检测到电池在热失控过程中产生了11.6 MPa和29.4 MPa的压力。目前较多研究者采用耐压密封罐体来进行电池热失控实验,通过测量罐内的压力变化,以推测电池热失控的产气速率和产气压[151]。例如,V. Somandepalli等[119]利用20 L密封燃烧室研究了电池热失控过程中的产气压力演化过程,发现SOC为100%和150%的LCO电池的最大压力分别为0.71 MPa和0.77 MPa。Zhao Chunpeng等[154-155]基于扩展容积绝热加速量热仪(Extended Vlume Accelerating Rate Calorimeter, EV-ARC)和压力罐分析了NCM电池的热失控行为演化,基于产生的压力和热能研究了老化程度和SOC对热危害的影响。C. Y. Wen等[166]、T. Y. Lu等[151]和W. C. Chen等[157]利用绝热量热仪(VSP2)测量了商用18650电池在不同SOC下的热行为和产气压变化,发现随着SOC的增大,LCO阴极材料电池的热失控最高温度、最大压力值、最大温升速率(dT/dt)max和最大压升速率(dp/dt)max都明显增大,但SOC对LFP电池的热失控特性影响较小,而且LFP电池的热失控剧烈程度远低于LCO电池。Wang Congjie等[167]发现当SOH≥80%且电流速率≥1C时,过充过程中电池将经历五个阶段:胀气、安全阀破裂、缓慢泄漏、强烈烟雾喷发和爆炸;但在较低的电流速率和SOH情况下电池不会发生爆炸。而且,热失控产生的最大压力随着电流速率C和SOH的增加而增大。Y. S. Duh等[168]和T. D. Hatchard等[169]比较了不同直径的圆柱形LFP电池的热失控行为,结果表明热失控曲线、最高温度和最大温升速率均与直径有关,而热失控起始温度和内部压力与直径无关。现有文献中关于热失控产气压力的分析总结见表3。
表2 典型文献中不同因素影响下的锂离子电池产气分析结果
Tab.2 Summary of available literature concerning the gas analyses of thermal runaway

文献电池参数触发条件排气总量/[mmol/(A·h)]①气体含量(体积分数③) 阴极型号SOC(%)SOH(%)CO2(%)CO(%)H2(%)CxHy(%)其他 [41]NCM18650100100200℃, 400 W29.97.315.313.1C7H14O2(0.4%), O2(0.24%) 60300℃, 400 W42.82.05.04.1C7H14O2(0.2%), O2(0.2%) 100300℃, 200 W24.99.922.416.4C7H14O2(0.5%), O2(0.15%) [57]LFP186500100过热50.093.51.82.72.1 5029.166.24.820.88.2 10029.148.39.129.413.1 11555.552.26.434.07.4 13052.755.87.730.16.4 NCA18650019.494.61.61.71.9 5046.933.839.917.58.8 10081.519.748.922.69.0 12083.920.848.723.57.0 14390.422.043.426.28.4 [85]LCO/NCM18650100100过热101.924.927.630.017.5 NCM99.341.213.030.815.0 LFP45.553.04.830.911.2 [106]NCM软包100100过热63.328.926.315.816.0EMC(3.0%) 过充115.018.833.326.114.0 针刺70.031.021.423.818.0 方形过热63.321.131.615.812.0DMC(2.0%), EMC(1.0%) 过充108.320.029.226.212.0 针刺71.725.623.320.913.0DMC(2.0%) [109]LCO18650100100过热1.8 g/(A·h)12.825.918.542.5O2(0.3%) NCM1.5 g/(A·h)24.330.510.534.6O2(0.1%) LFP0.3 g/(A·h)11.14.780.14.1 [115]NCAICR18650-32A100100过热(由上到下依次为首次排气、热失控和燃爆排气)1.282.2-2.4 15.4 0.0795.6-3.70.7 39.320.458.415.95.3 INR18650-35E1.8100--- 087.24-3.98.9 64.214.544.035.75.8 INR18650MJ10.5100--- 0.0798.1-0.91.1 61.49.837.243.29.9 [119]LCO方形50100过热380 mL/(A·h)32.03.630.034.0 100119 mL/(A·h)30.022.927.719.3 150286 mL/(A·h)20.924.529.724.0 [145]NCM软包100100过热63.3●②16.028.0●②EMC 9456.7●②28.028.0●②EMC, EC 8551.7●②24.024.0●②HF, EMC, EC, DMC, DEC 7645.0●②23.023.0●②
①排气总量单位默认为mmol/(A·h),其余单位在表格中另外注明。②“●”代表检测到了该种气体,具体含量未知。③气体含量单位默认为体积分数,其余单位在表格中另外注明。
表3 现有文献中关于热失控产气压力的总结
Tab.3 Summary of available literature concerning the pressure of thermal runaway
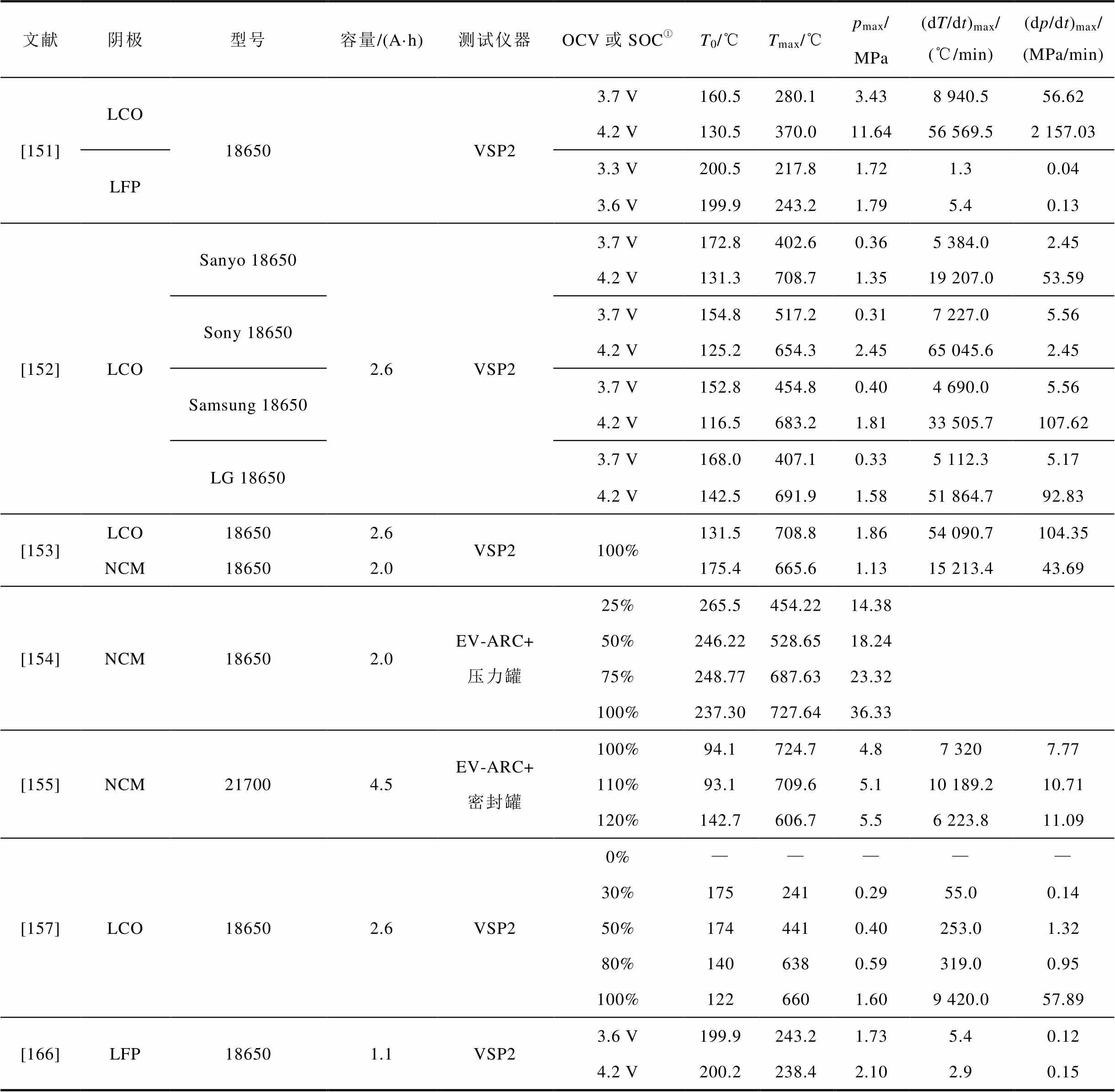
文献阴极型号容量/(A·h)测试仪器OCV或SOC①T0/℃Tmax/℃pmax/ MPa(dT/dt)max/(℃/min)(dp/dt)max/(MPa/min) [151]LCO18650VSP23.7 V160.5280.13.438 940.556.62 4.2 V130.5370.011.6456 569.52 157.03 LFP3.3 V200.5217.81.721.30.04 3.6 V199.9243.21.795.40.13 [152]LCOSanyo 186502.6VSP23.7 V172.8402.60.365 384.02.45 4.2 V131.3708.71.3519 207.053.59 Sony 186503.7 V154.8517.20.317 227.05.56 4.2 V125.2654.32.4565 045.62.45 Samsung 186503.7 V152.8454.80.404 690.05.56 4.2 V116.5683.21.8133 505.7107.62 LG 186503.7 V168.0407.10.335 112.35.17 4.2 V142.5691.91.5851 864.792.83 [153]LCO186502.6VSP2100%131.5708.81.8654 090.7104.35 NCM186502.0175.4665.61.1315 213.443.69 [154]NCM186502.0EV-ARC+压力罐25%265.5454.2214.38 50%246.22528.6518.24 75%248.77687.6323.32 100%237.30727.6436.33 [155]NCM217004.5EV-ARC+密封罐100%94.1724.74.87 3207.77 110%93.1709.65.110 189.210.71 120%142.7606.75.56 223.811.09 [157]LCO186502.6VSP20%————— 30%1752410.2955.00.14 50%1744410.40253.01.32 80%1406380.59319.00.95 100%1226601.609 420.057.89 [166]LFP186501.1VSP23.6 V199.9243.21.735.40.12 4.2 V200.2238.42.102.90.15
(续)

文献阴极型号容量/(A·h)测试仪器OCV或SOC①T0/℃Tmax/℃pmax/MPa(dT/dt)max/(℃/min)(dp/dt)max/(MPa/min) [168]LFPIFR 145000.9高压釜4.2 V222.1245.40.4512.0— A123 186501.14.2 V214.1286.30.2010.4 A123 266502.53.8 V208.5326.30.3890.0 A123 266502.54.2 V194.2395.50.45684.0 SONY 266503.03.8 V200.6403.00.43816.0 SONY 266503.04.2 V200.3457.70.421 392.0
①当表中数据表示开路电压(Open Circuit Voltage, OCV)时,单位为V;表示SOC时,用百分比来表示。
锂离子电池喷发气体是火灾形成的主要燃烧物质之一,研究其喷发过程可为热失控预警系统和灭火策略提供重要理论基础[170-172]。基于压力来分析电池的喷发过程可能比基于温度的分析更加准确[82]。首先,当安全阀开启时,密封腔室内的压力增加早于电池表面温度增加。其次,压力比温度稳定得更快,因为温度被许多因素直接影响[173],如电池产热、气体流动、气体液化和壁面散热等;而直接影响压力的因素则较少,比如电池喷发引起的气流(即分子的碰撞)等。因此,压力可以更加直接地反映电池的喷发过程,从而实现更加准确的热失控预警。F. A. Mier等[174]测量了通过安全阀的可压缩流的泄气激活压力和流量系数,并提供了安全阀激活后立即从盖子流出的纹影图像,但并没有对产气机制和通过通风口的多物种、多相流进行分析。王贺武等[172]基于高速摄影机研究了NCM方形电池在热失控过程中的初始喷发过程,观察到初始喷发首先呈现条状和锥形喷发,然后是较长时间的无定形喷发,之后是倒锥形喷发,但这种方法难以定量判断弱喷发过程。平平[173]基于布置在电池上方火区的温度传感器研究电池喷发过程,观察到电池着火期间,火区温度达到多个峰值,但由于喷发气流引起的复杂传热过程,仅仅根据温度很难准确地判断火灾喷发过程是否结束。上述研究对喷发过程的研究较为宏观,只研究了喷发时间和/或喷发次数。为了定量地评估电池喷发持续时间,ZhangYajun等[40]基于230 L耐压腔室研究了不同SOC下50 A·h棱形锂离子电池的喷发过程,将腔室压力上升速率与气室体积三次方根的乘积定义为喷发指标,基于这一指标,热失控喷发过程可分为超快、快和慢三个阶段。
如果能够实时地监测电池内部的压力变化,则对锂离子电池的状态检测可能更为准确,从而能够实现准确有效的事故预警[158-159,175]。Lei Boxia等[158]测量了商用18650 LMO电池的表面温度和电池内外部压力,结果如图10a~图10c所示。他们将引压线直接导入电池内部来测量内部压力。当排气发生时,电池内部的压力约为1.2 MPa,而外部压力在排气过程中最高可达到2.1 MPa。Qin Peng等[159]研究了NCM电池在排气前的内部压力演化过程,通过解耦裸电池中电解液蒸汽和化学反应产气引起的压力变化,得到了热失控过程中的气体生成量,推导出化学反应产气分压是安全阀开启的主要原因。他们还建立了产气率与产热率之间的关系,如图10d和图10e所示,发现在接近不可控温度时,温升速率与产气速率并不是线性关系。以上测量锂离子电池内压的方法都需要对电池外壳造成一定程度的损坏,可能导致电池性能下降。基于前人的研究,F. A. Mier等[175]提出了一种基于应变的无损内压检测方法,并通过实验验证了该方法的可行性和重复性。结果表明,应变测量可以更为直接地观察气体的生成,从而能够预测电池的排气故障,但低温条件下存在不稳定的应变行为,导致内部压力监测的误差较大。
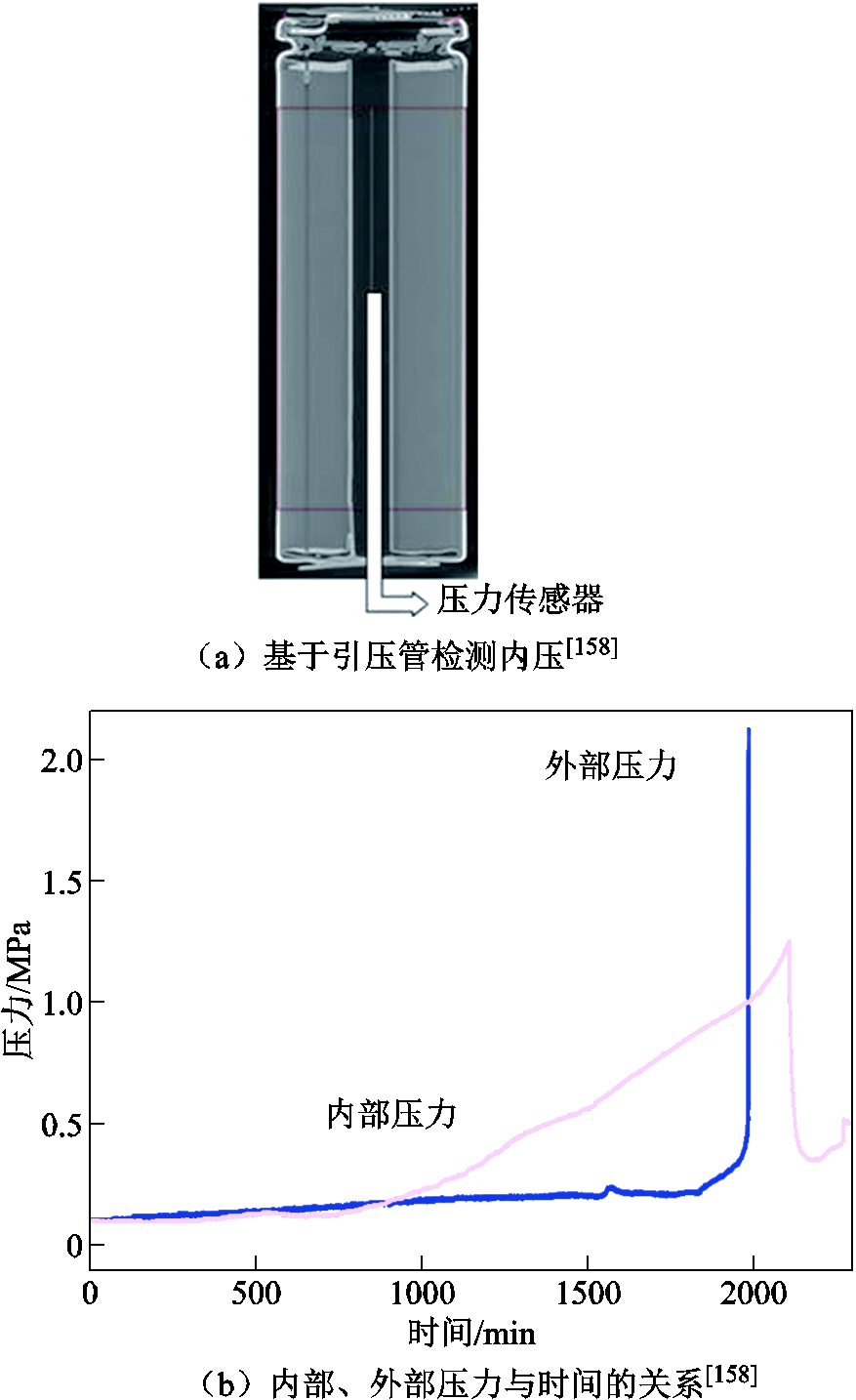
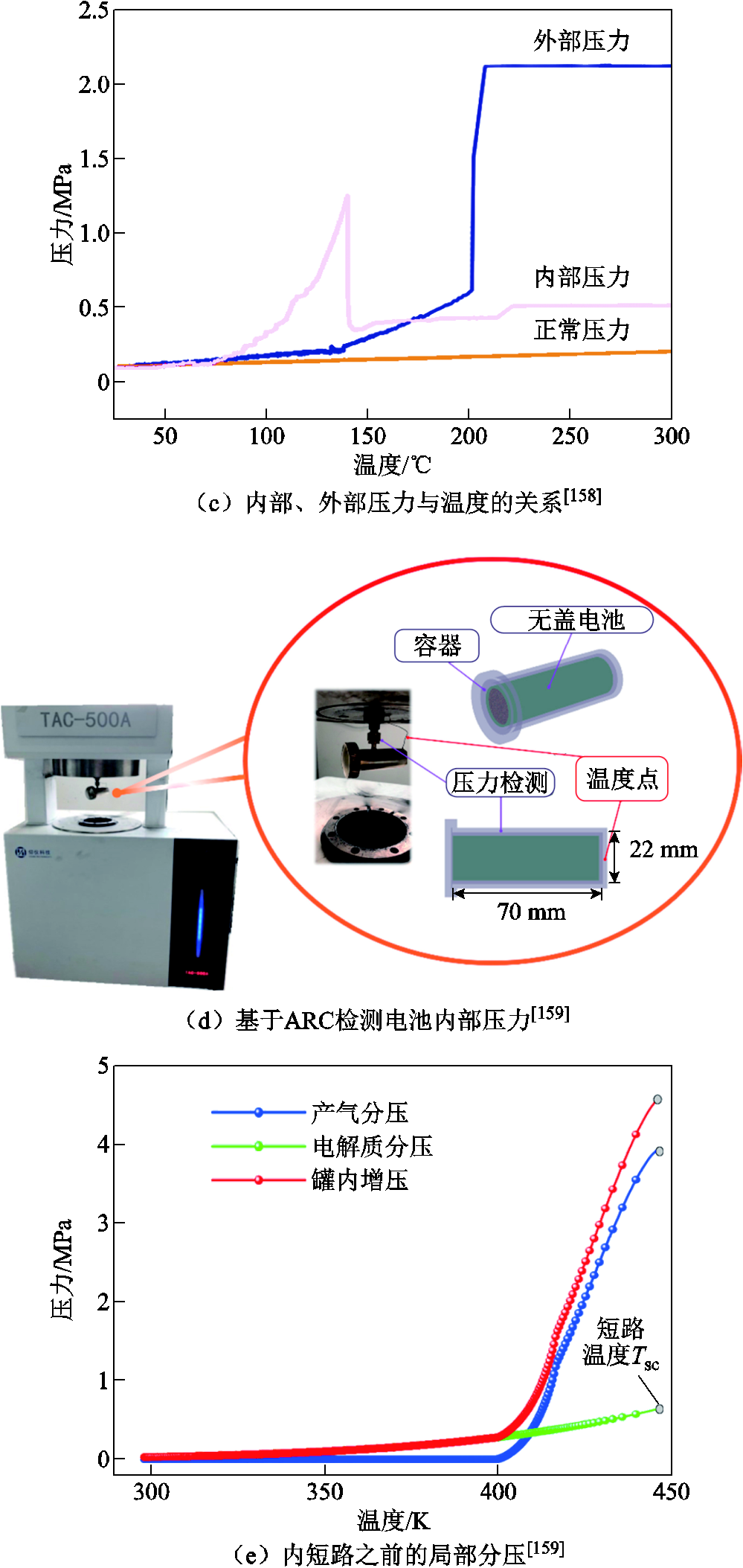
图10 检测电池内部压力[158-159]
Fig.10 Measure the internal pressure of the battery[158-159]
综上所述,目前分析锂离子电池喷发的研究一般基于密封腔室展开,输出参数常是电池温度、压力罐内部压力和产气组分,但这些研究难以确定气体温度,大多局限于对实验现象和压力峰值的描述,缺乏关于产气动力学模型方面的研究,因此很少涉及产气动力学参数和模型。
锂离子电池的喷发直接影响其外流动和燃烧特性[40],将锂离子电池的内部和外部事件耦合的方法主要有两种。第一种是基于零维(0 Dimension, 0D)集总模型来预测热反应和射流参数。P. T. Coman等[148-149]最先开发了热-流动耦合的集总参数模型来预测电池内的气体产生和压力升高,并量化了电池喷出物携带的能量。之后的模型大多遵循P. T. Coman等[148-149]的模型框架。例如,基于C. Y. Jhu等[176]测得的电池热分解过程的温度-压力数据,J. K. Ostanek等[160]在他们的数学模型中假设安全阀开启后的气体生成速率与该反应释放的热量成正比,对气体释放、排气机制和气体的可压缩流动过程进行建模分析,并建立了用于组件间传热的热阻网络。此外,模拟结果表明,电池顶空体积和外部表面传热系数的增加使得到达排气和热失控的时间随之增加,而且电解质蒸发速率的增加可以部分或完全抑制热失控的发生。然而,这种假设并不十分准确,因为电解液的蒸汽压也是腔室内压的重要组成部分,将密封罐内的压力增加归因于化学反应产气是不合理的。一些研究人员改进了经典模型,例如,J. Kim等[161]建立了一种随机多孔介质取代经典固体来模拟锂离子电池,基于安全阀底部多孔介质比例因子的变化来联系电池内部和外部。结果表明,该模型能够预测电池的气相反应和排气行为,但由于缺乏关于对流边界条件的准确估计,通过电池表面的热通量来预测内部相应的温度演变并不现实。P. J. Bugryniec等[162]提出了一种考虑增压排气和煨煮反应(Simmering Reactions, SR)的改进滥用模型,如图11所示,认为电池内部压力是由电解液/分解气体混合物的气泡点控制的,并与经典热失控模型[148-149]相比,提高了内部压力累积预测的精度。
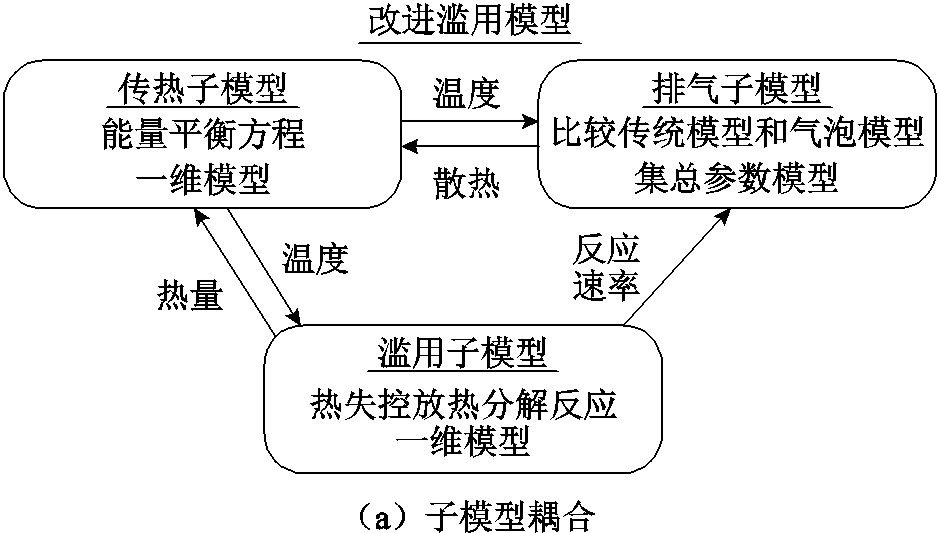
图11 改进滥用模型设计[162]
Fig.11 Advanced abuse model design[162]
以上模型提供了对热失控过程中产气机制的深入了解,但没有考虑排气后从电池中流出的多物种/多相流出物,在了解不同介质的气体产生和排放如何影响整个加热、排放和热失控过程中的温度演化及射流燃烧方面仍存在差距。为了深入研究锂离子电池产气过程和产热过程的内在联系,Mao Binbin等[177]基于EV-ARC和压力罐分析了不同SOC下的18650 NCM电池的产气过程,将热失控发生前的气体温度假设为电池和压力罐温度的均值。与Qin Peng等[159]得到的结论不同,Mao Binbin等发现在热失控过程中,产气速率与温升速率近似成正比例,并计算了气体生成反应的多阶段动力学参数。在此基础上,Mao Binbin等[178]开发了18650锂离子电池射流和火灾动力学的集总模型,通过动力学模型预测了电池的火焰高度和射流行为,描述了热滥用反应、压力积聚和排气特性之间的相关联系。但该模型并未考虑排气后的流场和由此产生的射流火灾的演变。Kong Depeng等[163]提出了一种基于流-固耦合传热和计算流体动力学的模型,以捕捉不同SOC下18650锂离子电池的热滥用、排气和燃烧下的电池温度和内部压力演化,采用集总参数模型对热失控反应和射流动力学进行了预测,并且对排气流量和燃烧进行了数值求解。结果表明,随着SOC的增加,热失控起始时刻会提前,射流速度峰值、射流火焰的峰值放热率和火焰高度也会随着SOC的增加而增加。
此外,单个锂离子电池的排气会对电池组造成一定的压力冲击。如果电池的安全阀出现故障,或者由于机械碰撞使得电池侧壁变形,可能导致侧壁断裂,从侧壁释放的气流会迅速将热能传递给电池组中的相邻电池,从而导致热失控的传播[179]。D. Mishra等[179]对锂离子电池喷发的热气流进行了非线性流体模拟,并研究了电池组几何参数对排气气体扩散的影响,结果表明,侧向输运和电芯间间隙输运所遇到的相对流动阻力是决定热失控传播是否发生的关键因素,而且排气孔的位置也是热失控传播到相邻电池的重要决定因素。表4总结了现有文献中关于锂离子电池热失控温度-压力演化行为方面的研究。
表4 锂离子电池热失控温度-压力演化特性研究总结
Tab.4 Summary of temperature-pressure evolution characteristics of thermal runaway of lithium ion batteries

文献模型维度考虑物理场结论局限性 [148]0D热-等熵流动方程量化了喷出物携带能量,证明了电解质和果冻卷的喷射散热对热失控温度有显著影响对化学反应产气过程分析不足,局限于产热方面的计算 [149]0D热-等熵流动方程能够预测电池内部的温度-压力行为和产气,建立了电解质溶剂DMC-温度的热力学性质表局限于单种电解质溶剂(DMC) [160]0D和热阻网络热-气体产生/排放模型模拟了气体的产生、排放以及可压缩流动过程,建立了用于组件间传热的热阻网络,可用于估算从排气到热失控的时间将罐内压力增加归因于化学反应产气,忽略了电解液挥发蒸汽 [161]0D排气、内压和气相动力学建立了考虑电池内的流体动力学和电池外的湍流的数值模型,研究了电池在不同SOC下的流动和热行为与产气量的函数关系将化学反应产气近似为六种气体,忽略了其他产气成分 [162]1D产热-内压-排气对P. T. Coman等[148-149]提出的经典模型进行改进,提高了预测精度,并分析了参数的不确定性热力学平衡近似为CO2-DMC混合物的相平衡,忽略了其余产气 [163]0D热失控-射流的耦合传热与流体动力学研究了不同SOC下的热失控分解反应、压力积聚和释放机制以及燃烧过程将化学反应产气近似为五种气体,忽略了其他产气成分;没有考虑火花喷射和辐射热对射流的影响 [179]3D非线性湍流研究了热失控释放气体携带热能的传播过程,以及电池组各几何参数对排气扩散的影响气体流动的温度和速度数据来源于前人研究,不具有普适性
锂离子电池在热失控过程中会发生一系列的连锁反应,使得电池内部温度升高,电解质溶剂挥发气体增加,电池内部压力大幅增加,直至安全阀打开或外壳破裂,释放出大量高度易燃的气体[10-12,15]。通常这种状态下的温度已经达到自燃温度,气体产物将开始燃烧或发生爆炸[40,100]。如果能够及时监测到热失控前锂离子电池的压力变化和排放气体,及时提供预警信号,可以为相关人员提供更长的逃生时间,避免严重的经济损失[19]。Feng Xuning等[11]基于能量释放图总结了热失控过程的连锁反应机制,并提出降低热失控危害的三级防护概念,在热失控发生之前提供被动防御和早期预警,减少热失控传播。A. Lecocq等[180]对电动汽车(包括锂离子电池组)和燃油车整车在外部火灾时的气体释放进行了时间分辨定量监测,燃烧特征见表5。基于FT-IR和在线气体分析仪实时检测了汽车燃烧过程中的气体产物,两种汽车火灾燃烧产物中的CO2、CO、总碳氢化合物(Total Hydrocarbons, THC)、NO、NO2、HCl和HCN的累计质量相似。由于现代汽车中引入了氟源,在传统内燃机汽车燃烧中也监测到了HF的排放,但因为锂离子电池组的燃烧,电动汽车中的HF累积质量更高。
表5 电动汽车和燃油车整车燃烧特征[180]
Tab.5 The combustion results of the electric vehicle[180]
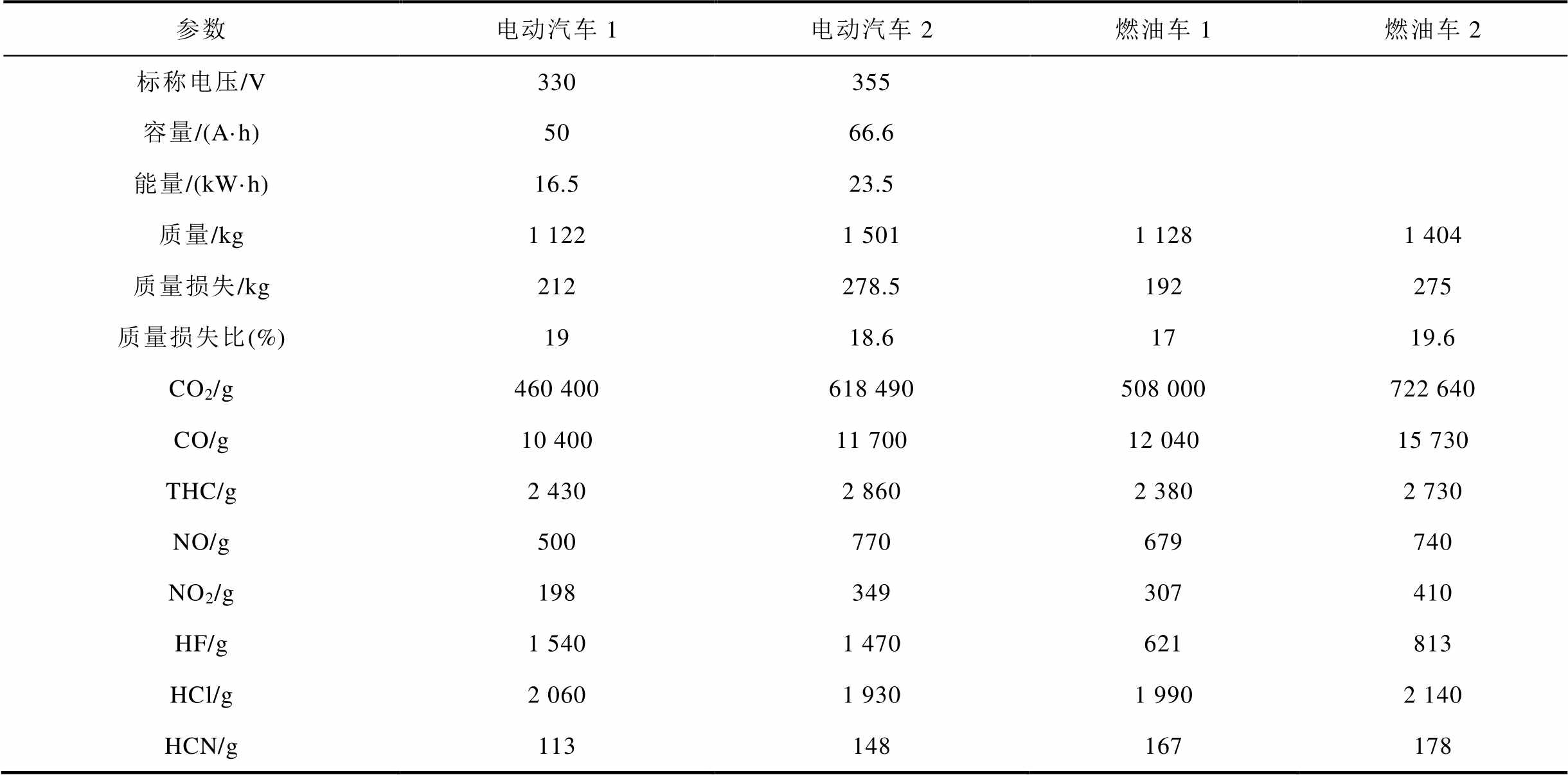
参数电动汽车1电动汽车2燃油车1燃油车2 标称电压/V330355 容量/(A·h)5066.6 能量/(kW·h)16.523.5 质量/kg1 1221 5011 1281 404 质量损失/kg212278.5192275 质量损失比(%)1918.61719.6 CO2/g460 400618 490508 000722 640 CO/g10 40011 70012 04015 730 THC/g2 4302 8602 3802 730 NO/g500770679740 NO2/g198349307410 HF/g1 5401 470621813 HCl/g2 0601 9301 9902 140 HCN/g113148167178
综上所述,基于电池释放的气体组分和内部压力的监测技术可以为热失控早期预警技术提供理论支撑。
基于上述分析可以看出,锂离子电池热失控产气的主要成分为CO、H2、CO2和碳氢化合物等[181],研究人员基于这一现象开展了一系列基于单一气体的热失控早期预警技术[10,182-187],其中,基于H2和电解质挥发气体的预警研究引起了广泛关注。例如,黄峥等[182]基于FT-IR和H2探头实时监测了86 A·h LFP电池热失控产气过程,排出气体的主要成分为CO2和H2,分别占比30.15%和39.5%,其中H2排放的时间要早于其他气体成分。Jin Yang等[10]开发了一种基于H2检测微量锂枝晶的方法,原理如图12所示,并通过LFP过充实验得到了验证。后续在电池舱中布置的H2、CO、CO2、HCl、HF和SO2气体传感器最先检测到H2,且捕获H2的时间比监测到冒烟和着火分别早639 s和769 s。如果检测到H2后立即采取措施,在无烟无火的情况下,完全可以阻止锂枝晶的生长,抑制热失控的进程。随着锂离子电池的循环使用,其内部组件逐渐老化,需要根据电池的实际使用情况制定热失控预警技术,而且,在不同SOC的热失控下要选择适合的特征气体成分和含量建立预警机制。例如,N. E. Galushkin等[183]发现石墨阳极循环过程中会逐渐积累氢,氢原子的重新组合产生了强烈的放热反应,使得电池的热失控起始温度显著降低,不同循环后热失控反应释放出的H2总量也不同,如图13所示。
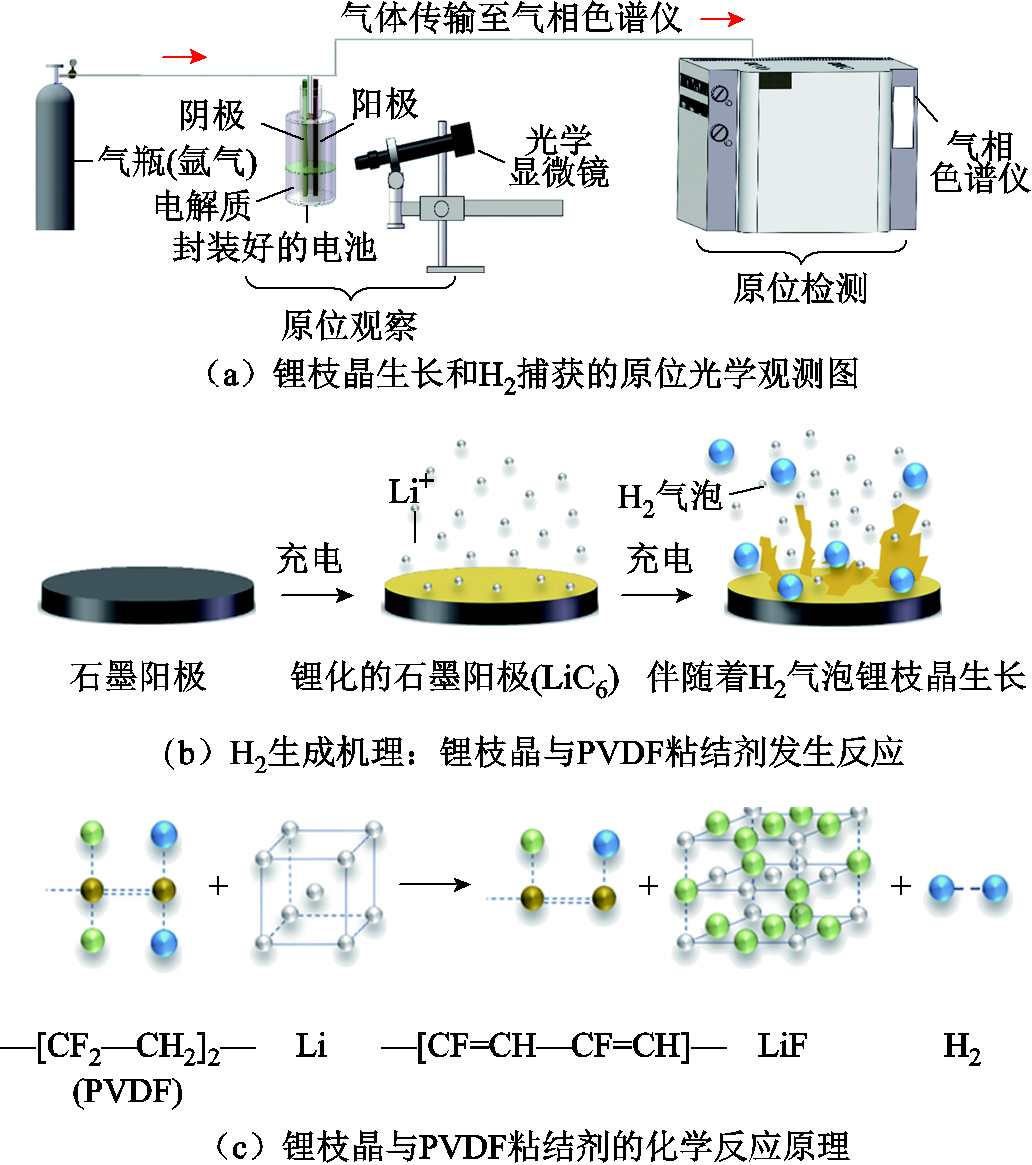
图12 H2捕获法检测锂枝晶生长原理[10]
Fig.12 Schematic diagram of the detection of lithium dendrite growth by the H2 capture method[10]
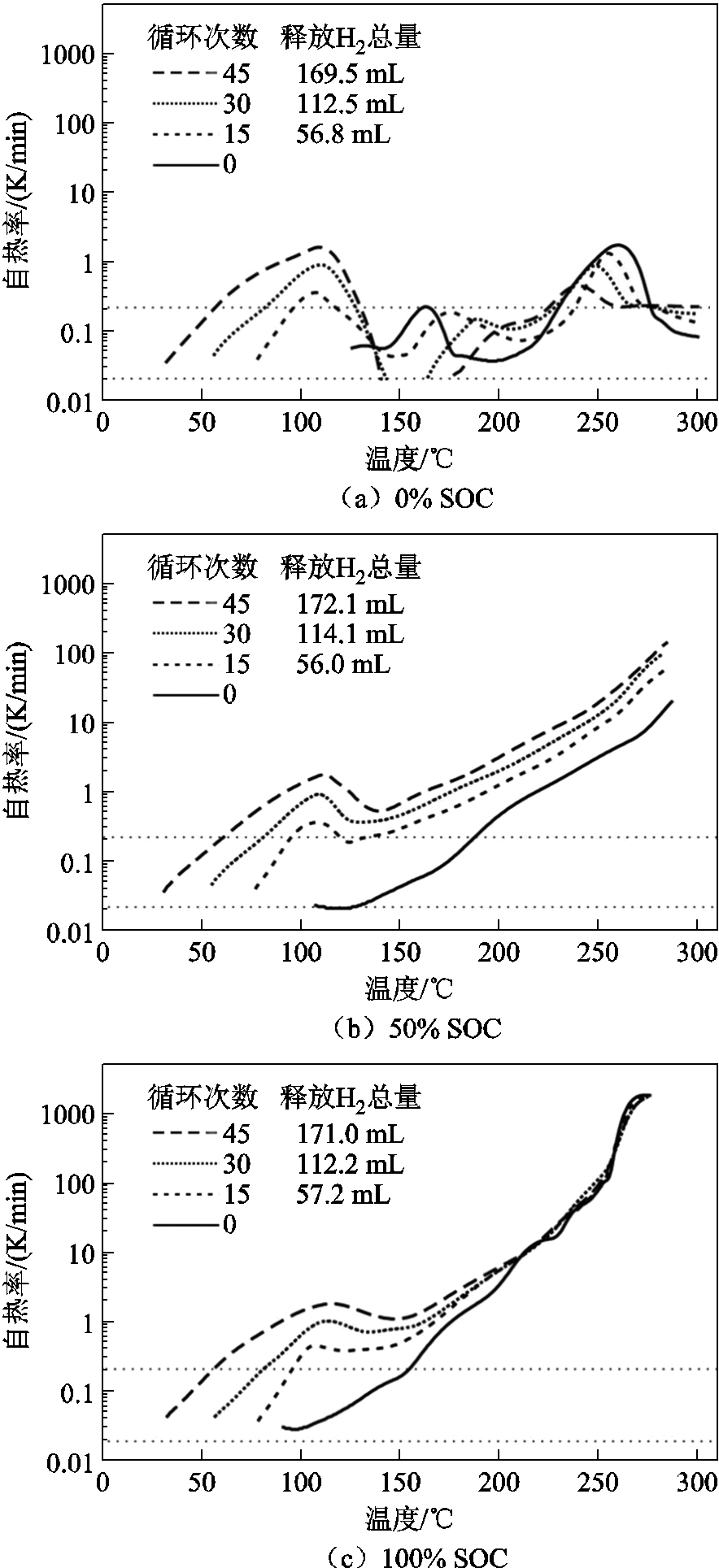
图13 不同循环次数后对18650 NCM电池进行热失控实验[183]
Fig.13 Thermal runaway tests of 18650 NCM cells after different cycles[183]
针对电解液挥发气体,研究人员也开发了大量在线监测装置[184]。例如,M. Wenger等[184]开发了一种专用于检测电解质挥发气体的新型微机电系统(Micro-Electro-Mechanical System, MEMS)气体传感器,并测试了该气体传感器在漏液和过充情况下故障预警的适用性。结果表明,气体传感器能够在漏液发生35 s后发出预警信号。而在过充测试中,预警结果随充电电流的变化而变化,随着电流速率的增加,热失控前的预警时间随之减小,但即使在12C(60 A)电流速率下,气体传感器仍可提前40 s提供预警。因此,如果能够在传感器内阻明显下降时及时采取措施,如停止充电,则可以避免热失控事故的发生。美国Nexceris公司也公开了一项基于SnO2陶瓷半导体气体传感器的锂离子电池热失控预警系统的专利,该传感器可以监测到10-6%级别的电解液蒸汽[185]。杨启帆等[186]基于对锂离子电池在过充、漏液、高温和短路故障下的产气分析,研究了基于挥发性有机物(Volatile Organic Compounds, VOC)的故障诊断技术,并且适用于不同的SOC下的锂离子电池。Lu Yang等[187]基于离子导电金属有机框架(Ionically Conductive Metal-Organic Frameworks, IC-MOF)开发了一种新型气体传感器,如图14所示,能够在几秒钟之内快速检测到10-6%级别的漏液,而漏液的电池电压在几个小时内几乎保持与原始电池相同的水平,表明该传感器具有数小时的预警能力。
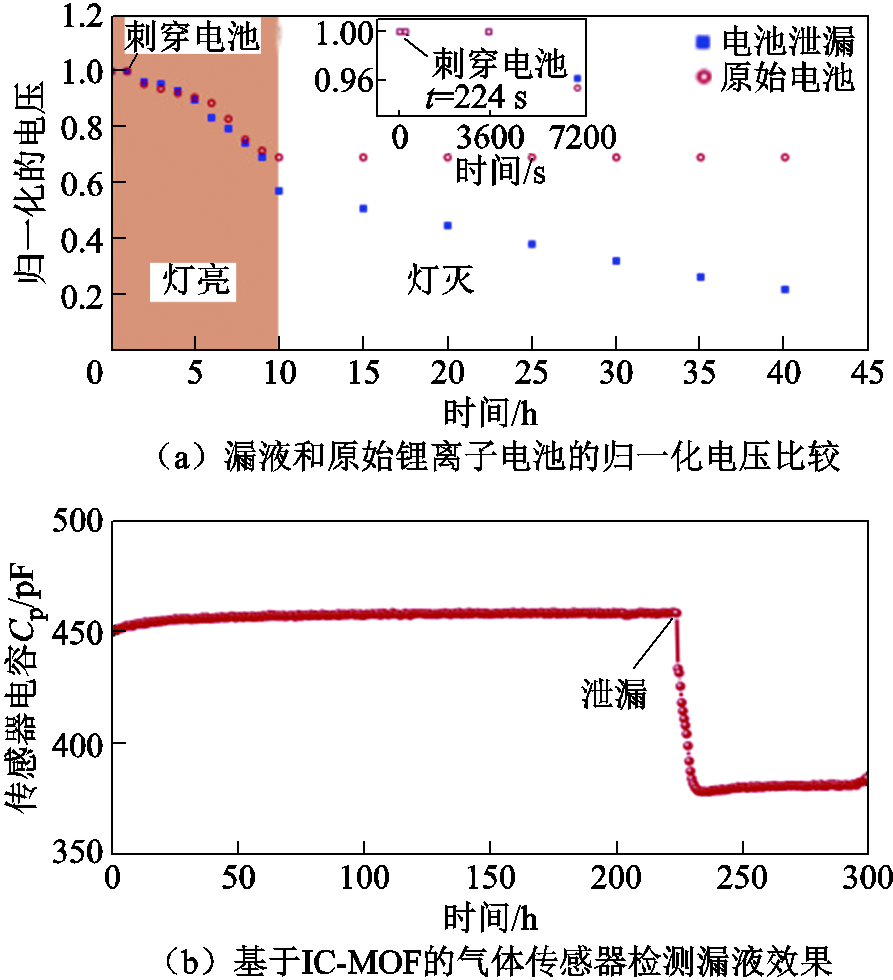
图14 基于IC-MOF的气体传感器实时监测锂离子电池漏液[187]
Fig.14 IC-MOF-based gas sensor for real-time monitoring of lithium-ion battery leakage[187]
此外,Cai Ting等[188]基于COMSOL建立了锂离子电池模组热失控早期检测仿真模型,并且证明基于CO2气体的传感速度要比传统的电池表面温度传感更迅速,可以在发生热传播之前及时检测到电芯的热失控。Sun Jie等[125]利用多气体监测仪对18650电池安全阀冲爆后的CO气体进行检测,通过CO的含量突变判断电池是否处于热失控状态,可以实时监测电池安全状态。而Liao Zhenghai等[118]基于GASTEC气体检测管检测了热失控后HF气体的含量,认为可以基于HF的气敏技术建立预警机制。
单一气体的预警效果有限,因此,研究人员开发了基于两种或多种气体的热失控早期预警机制,并结合现有BMS制定了多级预警策略[189-192]。例如,王志荣等[189]开发了一套基于气体报警的锂离子电池高温预警系统,一旦特征气体CO和H2的含量达到120×10-4%时,装置即发出报警信号。A. Raghavan等[190]通过在锂离子电池中内置光纤(Fiber Optic, FO)传感器,以监测电池内游离或溶解气体含量的变化,选择CO2和碳氢化合物两种气体来反映电池的安全状态。D. Hill等[191]利用新型气体传感器检测测试箱体内或气体出口处的气体,结果表明该传感器在热失控发生前10 min就提供了预警信号,排气组分包括CO、CH4、C2H4、C2H6、C3H8、C3H6O3、C5H10O3、HF及其他VOC气体。此外,王铭民等[192]基于对LFP电池过充实验的结果,如图15所示,认为CO2、CO和H2可作为一级预警气体,HF和HCl作为二级预警气体。图中EX(LEL)表示以爆炸下限百分比表示的烃类气体。

图15 基于气体的热失控分级预警[192]
Fig.15 Gas-based thermal runaway classification warning[192]
基于单一传感器无法满足实际工程应用的检测需求,因此,基于多参量共同监测的融合机制对锂离子电池故障的识别更加有效和及时[105,193-194]。王春力等[194]将温度与CO气体信号结合作为锂离子电池热失控的预警,分析了储能电站多级预警和防控机制及其安全联动策略,从而极大地提升了储能系统运行的安全性。而且,电池内部的压力可以用来反映其内部状态。例如,Wang Qian等[195]证明了钛酸锂电池内压的升高与下降可分别表征充电过程与放电过程。H. K. Kim等[150]通过测量镍氢电池的内部压力来估计其运行状态,并证实了电池的内部压力与电池运行时间的一对一关系。因此,可以建立基于融合多参量的电池故障预警方案。例如,S. Koch等[105]基于已确定的外部加热和针刺条件下的热失控行为,采用电压、气体、烟雾、温度、压力等多传感器联用共同监测NCM锂离子电池热失控过程,从检测速度、信号清晰度和传感器部署的可行性方面给出了不同传感器的评价,见表6。表中,信号清晰度为信号接近阶跃函数的程度及实现的难易程度;传感器的可行性用来评估部署的难易程度;评价分为三个等级:好(+)、中(0)、差(-)。从表6可以看出,SnO2半导体气体传感器和压力传感器对于热失控的预警速度最快,要早于温度、电压等常规监测手段。邓孝元[193]基于商用传感器研发了热失控温度、气体、火焰、气压和烟雾监测系统,能够对热失控进行实时预警。此外,A. Ganguli等[31-32]在电池内部嵌入光纤传感器,基于内部压力的变化预测SOC和SOH,进而实现热失控的预警,但该方法成本较高,难以推广。综上所述,本节从测试参数、测试手段和有效性方面总结了现有文献中基于气体的热失控预警方案,见表7。
表6 对传感器的评价[105]
Tab.6 Evaluation of sensors[105]
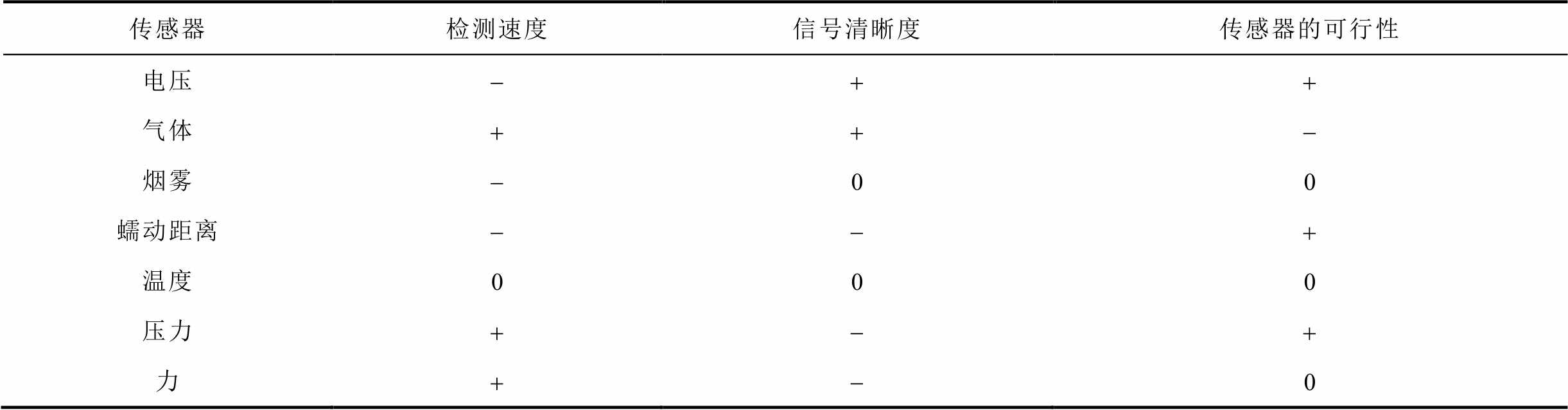
传感器检测速度信号清晰度传感器的可行性 电压-++ 气体++- 烟雾-00 蠕动距离--+ 温度000 压力+-+ 力+-0
表7 现有文献中基于气体的热失控预警研究总结
Tab.7 Summary of gas-based thermal runaway early warning studies in existing literature
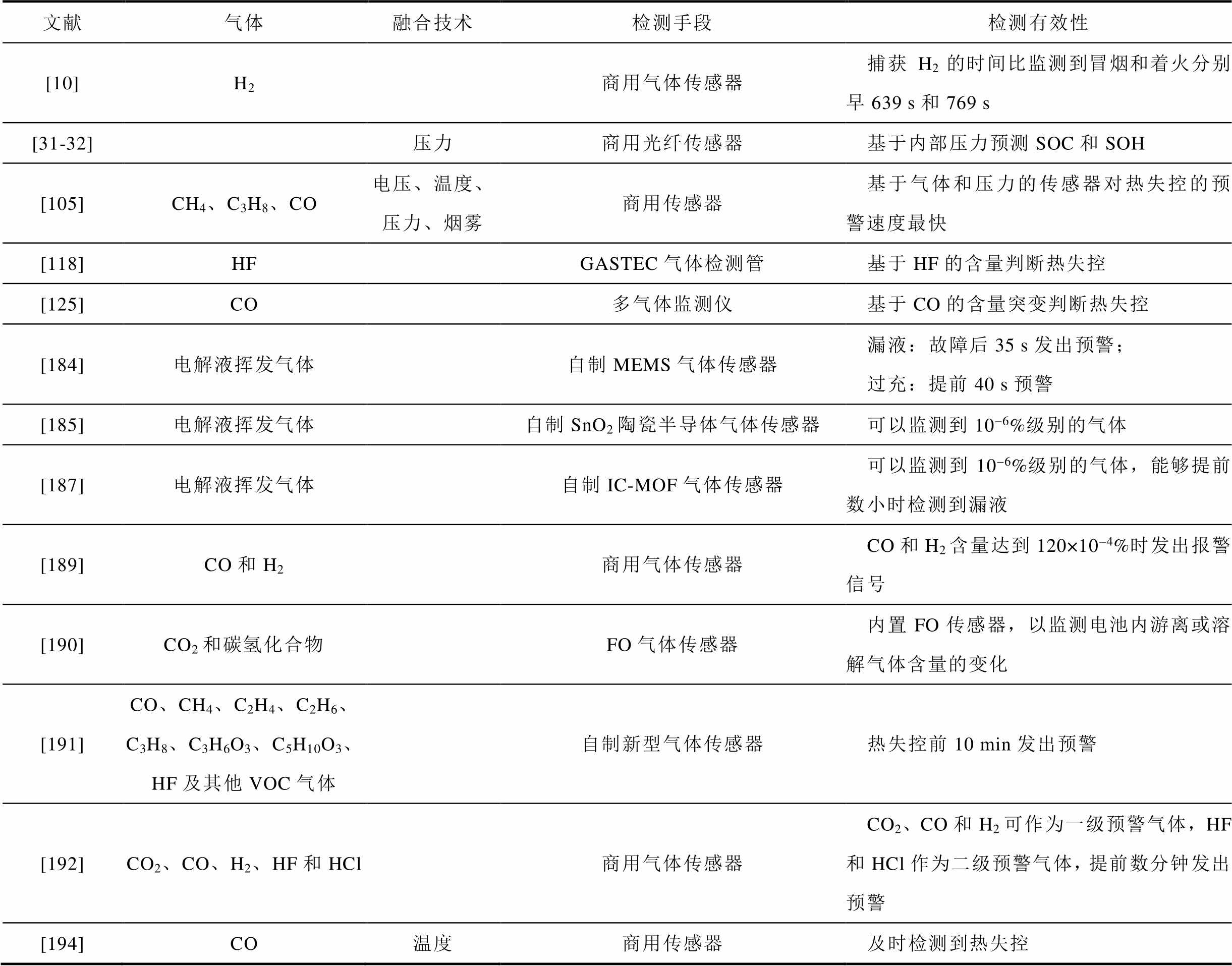
文献气体融合技术检测手段检测有效性 [10]H2商用气体传感器捕获H2的时间比监测到冒烟和着火分别早639 s和769 s [31-32]压力商用光纤传感器基于内部压力预测SOC和SOH [105]CH4、C3H8、CO电压、温度、压力、烟雾商用传感器基于气体和压力的传感器对热失控的预警速度最快 [118]HFGASTEC气体检测管基于HF的含量判断热失控 [125]CO多气体监测仪基于CO的含量突变判断热失控 [184]电解液挥发气体自制MEMS气体传感器漏液:故障后35 s发出预警;过充:提前40 s预警 [185]电解液挥发气体自制SnO2陶瓷半导体气体传感器可以监测到10-6%级别的气体 [187]电解液挥发气体自制IC-MOF气体传感器可以监测到10-6%级别的气体,能够提前数小时检测到漏液 [189]CO和H2商用气体传感器CO和H2含量达到120×10-4%时发出报警信号 [190]CO2和碳氢化合物FO气体传感器内置FO传感器,以监测电池内游离或溶解气体含量的变化 [191]CO、CH4、C2H4、C2H6、C3H8、C3H6O3、C5H10O3、HF及其他VOC气体自制新型气体传感器热失控前10 min发出预警 [192]CO2、CO、H2、HF和HCl商用气体传感器CO2、CO和H2可作为一级预警气体,HF和HCl作为二级预警气体,提前数分钟发出预警 [194]CO温度商用传感器及时检测到热失控
当遭遇电、热和机械滥用时,锂离子电池极易发展为热失控,进而引发火灾和爆炸事件,对人员安全和社会经济造成了极大的威胁,也限制了其在储能电站和电动汽车领域的进一步发展。因此,亟须开发和设计出快速且准确的锂离子电池热失控早期预警装置,为人员逃生和故障处理提供充足的时间。现有BMS基于电压、温度等特征信号可能无法及时识别出热失控,而基于气体的热失控早期预警技术可能要比传统的电、热信号更加灵敏,从而能够实现锂离子电池热失控的早期预警。总结现有文献可以得到如下结论:
1)锂离子电池热失控过程中主要排出的气体产物为CO2、CO、H2、碳氢化合物和电解质溶剂挥发物,此外,还可能包含少量的氟化物。
2)热失控触发条件及锂离子电池的组成材料、包装型号、SOC和SOH都会对热失控产气成分和含量产生影响。一般来说,过充诱导的热失控要比其他滥用下的热失控产气总量更多、排气速率更快、毒性危害更大;在常用的锂离子电池阴极材料中,LFP的释氧能力较弱,不同阴极材料热失控产气的危害性由高到低为:NCA>LCO/NCM>NCM>LFP;热失控产气组分不受电池容量的影响,但外壳包装对电池产气的影响较大,软包电池通常发生一次排气事件,而硬壳电池可能会经历2次甚至3次排气,而且软包电池会释放出更多HF有毒气体;随着SOC的增加,热失控产气总量和气体种类增多,且产气中CO2的含量降低,但CO和H2可燃有毒气体组分占比随之增加,在100% SOC下的产气会导致最严重的毒性,此外,在50% SOC下表现出最低的燃爆风险;随着SOH的降低,锂离子电池热失控释放气体总量也会随之减小,但可燃气体占比将会增加,且老化后的电池产气行为则不存在SOC的依赖性。
3)锂离子电池在热失控过程中的压力变化可以反映电池内部状态,内部压力的升高是由化学反应产气和电解液挥发蒸汽引起的,其中化学反应产气导致的压力增加主导了安全阀的打开。有部分学者认为热失控过程中的产气率与产热率之间为线性关系,但仍存在争议,需要进一步的研究。为了深入分析产气机制,研究人员结合化学反应动力学、计算流体学等,建立数学模型对产气过程进行模拟,从而预测了温度变化、压力积聚、排气流量及射流燃烧。综上所述,基于压力分析电池热失控的喷发过程可能要比基于温度的方法更为准确,从而实现及早且准确的热失控早期预警。
4)研究人员开发了基于气体的故障诊断技术,为锂离子电池的热失控提供早期预警信号,并结合现有BMS测量的电、热信号构建融合技术体系,从而实现储能电站或电动汽车的多级预警和防控机制。常用于预警的气体为H2、CO、CO2,近年来,电解质挥发气体由于在热失控过程中最先释放出来而引起了广泛关注,并被证明可提前数小时发出报警信号。
但目前基于气体和压力的热失控预警仍有许多不足:
1)针对气体传感器的热失控预警研究中,都是将气体传感器放置于监测的最佳位置。在实际应用中,由于电池系统的机械限制和系统中使用的气体传感器数量有限,气体传感器不太可能总是安装在故障电池附近,气体传感器的响应可能并不十分理想。因此,必须针对电池系统设计特定的气体传感预警装置。
2)现有半导体气体传感器存在气体交叉干扰、检测精度受限、气体传感器中毒等问题,亟须研制MEMS光声光谱仪、红外光谱仪等便携式气体传感器。
3)针对基于气压的热失控预警研究,需要改进锂离子电池的封装工艺,保证嵌入探头后锂离子电池的性能不受影响,同时,需要提高传感器探头的耐高温和分辨率能力。此外,可以将基于气体传感器和气压的检测技术与现有BMS检测技术结合起来,开发更为精确和灵敏的锂离子电池热失控早期预警技术。
参考文献
[1] 吴赋章, 杨军, 林洋佳, 等. 考虑用户有限理性的电动汽车时空行为特性[J]. 电工技术学报, 2020, 35(7): 1563-1574.
Wu Fuzhang, Yang Jun, Lin Yangjia, et al. Research on spatiotemporal behavior of electric vehicles considering the users’ bounded rationality[J]. Transactions of China Electrotechnical Society, 2020, 35(7): 1563-1574.
[2] 武龙星, 庞辉, 晋佳敏, 等. 基于电化学模型的锂离子电池荷电状态估计方法综述[J]. 电工技术学报, 2022, 37(7): 1703-1725.
Wu Longxing, Pang Hui, Jin Jiamin, et al. A review of SOC estimation methods for lithium-ion batteries based on electrochemical model[J]. Transactions of China Electrotechnical Society, 2022, 37(7): 1703-1725.
[3] 黄凯, 丁恒, 郭永芳, 等. 基于数据预处理和长短期记忆神经网络的锂离子电池寿命预测[J]. 电工技术学报, 2022, 37(15): 3753-3766.
Huang Kai, Ding Heng, Guo Yongfang, et al. Prediction of remaining useful life of lithium-ion battery based on adaptive data preprocessing and long short-term memory network[J]. Transactions of China Electrotechnical Society, 2022, 37(15): 3753-3766.
[4] 肖迁, 穆云飞, 焦志鹏, 等. 基于改进LightGBM的电动汽车电池剩余使用寿命在线预测[J]. 电工技术学报, 2022, 37(17): 4517-4527.
Xiao Qian, Mu Yunfei, Jiao Zhipeng, et al. Improved LightGBM based remaining useful life prediction of lithium-ion battery under driving conditions[J]. Transactions of China Electrotechnical Society, 2022, 37(17): 4517-4527.
[5] 肖迁, 焦志鹏, 穆云飞, 等. 基于LightGBM的电动汽车行驶工况下电池剩余使用寿命预测[J]. 电工技术学报, 2021, 36(24): 5176-5185.
Xiao Qian, Jiao Zhipeng, Mu Yunfei, et al. LightGBM based remaining useful life prediction of electric vehicle lithium-ion battery under driving conditions[J]. Transactions of China Electrotechnical Society, 2021, 36(24): 5176-5185.
[6] Yang Mengjie, Ye Yijun, Yang Aijun, et al. Comparative study on aging and thermal runaway of commercial LiFePO4/graphite battery undergoing slight overcharge cycling[J]. Journal of Energy Storage, 2022, 50: 104691.
[7] 薛明, 杨庆新, 章鹏程, 等. 无线电能传输技术应用研究现状与关键问题[J]. 电工技术学报, 2021, 36(8): 1547-1568.
Xue Ming, Yang Qingxin, Zhang Pengcheng, et al. Application status and key issues of wireless power transmission technology[J]. Transactions of China Electrotechnical Society, 2021, 36(8): 1547-1568.
[8] Yoshino A. Development of the lithium-ion battery and recent technological trends[M]//Pistoia. Lithium-Ion Batteries. Amsterdam: Elsevier, 2014: 1-20.
[9] Market.Us. Lithium Ion Battery Market is Slated to be Worth USD 307.8 Billion by 2032[EB/OL]. (2023-02-28).https://www.globenewswire.com/en/news-release/ 2023/02/28/2617605/0/en/Lithium-Ion-Battery-Market- is-Slated-to-be-Worth-USD-307-8-Billion-by-2032-Market-Us.html.
[10] Jin Yang, Zheng Zhikun, Wei Donghui, et al. Detection of micro-scale Li dendrite via H2 gas capture for early safety warning[J]. Joule, 2020, 4(8): 1714-1729.
[11] Feng Xuning, Ouyang Minggao, Liu Xiang, et al. Thermal runaway mechanism of lithium ion battery for electric vehicles: a review[J]. Energy Storage Materials, 2018, 10: 246-267.
[12] Wang Qingsong, Mao Binbin, Stoliarov S I, et al. A review of lithium ion battery failure mechanisms and fire prevention strategies[J]. Progress in Energy and Combustion Science, 2019, 73: 95-131.
[13] Electric Power Research Institute. BESS Failure Event Database[DB/OL]. (2022-08-17)[2023-03-02]. https:// storagewiki.epri.com/index.php/BESS_Failure_Event_Database.
[14] 电动观察. 2021电动汽车安全年度报告[EB/OL]. (2022-01-06)[2023-03-02]. https://baijiahao.baidu. com/ s?id=1721164 341983283279&wfr=spider&for=pc.
[15] Feng Xuning, Ren Dongsheng, He Xiangming, et al. Mitigating thermal runaway of lithium-ion batteries[J]. Joule, 2020, 4(4): 743-770.
[16] Liu Xiang, Ren Dongsheng, Hsu Hungjen, et al. Thermal runaway of lithium-ion batteries without internal short circuit[J]. Joule, 2018, 2(10): 2047-2064.
[17] 牛志远, 姜欣, 谢镔, 等. 电动汽车过充燃爆事故模拟及安全防护研究[J]. 电工技术学报, 2022, 37(1): 36-47, 57.
Niu Zhiyuan, Jiang Xin, Xie Bin, et al. Study on simulation and safety protection of electric vehicle overcharge and explosion accident[J]. Transactions of China Electrotechnical Society, 2022, 37(1): 36-47, 57.
[18] 庞辉, 郭龙, 武龙星, 等. 考虑环境温度影响的锂离子电池改进双极化模型及其荷电状态估算[J]. 电工技术学报, 2021, 36(10): 2178-2189.
Pang Hui, Guo Long, Wu Longxing, et al. An improved dual polarization model of Li-ion battery and its state of charge estimation considering ambient temperature[J]. Transactions of China Electrotechnical Society, 2021, 36(10): 2178-2189.
[19] Duan Jian, Tang Xuan, Dai Haifeng, et al. Building safe lithium-ion batteries for electric vehicles: a review[J]. Electrochemical Energy Reviews, 2020, 3(1): 1-42.
[20] Liao Zhenghai, Zhang Shen, Li Kang, et al. A survey of methods for monitoring and detecting thermal runaway of lithium-ion batteries[J]. Journal of Power Sources, 2019, 436: 226879.
[21] Xia Bing, Mi C. A fault-tolerant voltage measurement method for series connected battery packs[J]. Journal of Power Sources, 2016, 308: 83-96.
[22] Xia Bing, Nguyen T, Yang Jufeng, et al. The improved interleaved voltage measurement method for series connected battery packs[J]. Journal of Power Sources, 2016, 334: 12-22.
[23] Xia Bing, Shang Yunlong, Nguyen T, et al. A correlation based fault detection method for short circuits in battery packs[J]. Journal of Power Sources, 2017, 337: 1-10.
[24] Grandjean T, Barai A, Hosseinzadeh E, et al. Large format lithium ion pouch cell full thermal characterisation for improved electric vehicle thermal management[J]. Journal of Power Sources, 2017, 359: 215-225.
[25] Parhizi M, Ahmed M B, Jain A. Determination of the core temperature of a Li-ion cell during thermal runaway[J]. Journal of Power Sources, 2017, 370: 27-35.
[26] Raijmakers L H J, Danilov D L, van Lammeren J P M, et al. Sensorless battery temperature measurements based on electrochemical impedance spectroscopy[J]. Journal of Power Sources, 2014, 247: 539-544.
[27] Raijmakers L H J, Danilov D L, van Lammeren J P M, et al. Non-zero intercept frequency: an accurate method to determine the integral temperature of Li-ion batteries[J]. IEEE Transactions on Industrial Electronics, 2016, 63(5): 3168-3178.
[28] Srinivasan R, Demirev P A, Carkhuff B G. Rapid monitoring of impedance phase shifts in lithium-ion batteries for hazard prevention[J]. Journal of Power Sources, 2018, 405: 30-36.
[29] Srinivasan R, Carkhuff B G, Butler M H, et al. Instantaneous measurement of the internal temperature in lithium-ion rechargeable cells[J]. Electrochimica Acta, 2011, 56(17): 6198-6204.
[30] Lyu Nawei, Jin Yang, Xiong Rui, et al. Real-time overcharge warning and early thermal runaway prediction of Li-ion battery by online impedance measurement[J]. IEEE Transactions on Industrial Electronics, 2022, 69(2): 1929-1936.
[31] Raghavan A, Kiesel P, Sommer L W, et al. Embedded fiber-optic sensing for accurate internal monitoring of cell state in advanced battery management systems part 1: cell embedding method and performance[J]. Journal of Power Sources, 2017, 341: 466-473.
[32] Ganguli A, Saha B, Raghavan A, et al. Embedded fiber-optic sensing for accurate internal monitoring of cell state in advanced battery management systems part 2: internal cell signals and utility for state estimation[J]. Journal of Power Sources, 2017, 341: 474-482.
[33] Fortier A, Tsao M, Williard N D, et al. Preliminary study on integration of fiber optic Bragg grating sensors in Li-ion batteries and in situ strain and temperature monitoring of battery cells[J]. Energies, 2017, 10(7): 838.
[34] Kennedy R W, Marr K C, Ezekoye O A. Gas release rates and properties from Lithium Cobalt Oxide lithium ion battery arrays[J]. Journal of Power Sources, 2021, 487: 229388.
[35] Diaz F, Wang Y, Weyhe R, et al. Gas generation measurement and evaluation during mechanical processing and thermal treatment of spent Li-ion batteries[J]. Waste Management, 2019, 84: 102-111.
[36] Larsson F, Andersson P, Blomqvist P, et al. Toxic fluoride gas emissions from lithium-ion battery fires[J]. Scientific Reports, 2017, 7(1): 10018.
[37] Fernandes Y, Bry A, de Persis S. Identification and quantification of gases emitted during abuse tests by overcharge of a commercial Li-ion battery[J]. Journal of Power Sources, 2018, 389: 106-119.
[38] Kong Weihe, Li Hong, Huang Xuejie, et al. Gas evolution behaviors for several cathode materials in lithium-ion batteries[J]. Journal of Power Sources, 2005, 142(1-2): 285-291.
[39] Kumai K, Miyashiro H, Kobayashi Y, et al. Gas generation mechanism due to electrolyte decomposition in commercial lithium-ion cell[J]. Journal of Power Sources, 1999, 81-82: 715-719.
[40] Zhang Yajun, Wang Hewu, Li Weifeng, et al. Quantitative analysis of eruption process of abused prismatic Ni-rich automotive batteries based on in-chamber pressure[J]. Journal of Energy Storage, 2020, 31: 101617.
[41] Chen Shichen, Wang Zhirong, Wang Jinghong, et al. Lower explosion limit of the vented gases from Li-ion batteries thermal runaway in high temperature condition[J]. Journal of Loss Prevention in the Process Industries, 2020, 63: 103992.
[42] Sturk D, Rosell L, Blomqvist P, et al. Analysis of Li-ion battery gases vented in an inert atmosphere thermal test chamber[J]. Batteries, 2019, 5(3): 61.
[43] Cummings S, Swartz N. Off-gas monitoring for lithium ion battery health and safety[R]//Wright Patterson AFB: Power Sources Committee Meeting, 2017.
[44] 廖正海, 张国强. 锂离子电池热失控早期预警研究进展[J]. 电工电能新技术, 2019, 38(10): 61-66.
Liao Zhenghai, Zhang Guoqiang. Progress on early warning technology for thermal runaway of lithium-ion battery[J]. Advanced Technology of Electrical Engineering and Energy, 2019, 38(10): 61-66.
[45] Wang Qingsong, Jiang Lihua, Yu Yan, et al. Progress of enhancing the safety of lithium ion battery from the electrolyte aspect[J]. Nano Energy, 2019, 55: 93-114.
[46] Feng Xuning, Zheng Siqi, Ren Dongsheng, et al. Investigating the thermal runaway mechanisms of lithium-ion batteries based on thermal analysis database[J]. Applied Energy, 2019, 246: 53-64.
[47] Aurbach D, Zaban A, Gofer Y, et al. Recent studies of the lithium-liquid electrolyte interface Electrochemical, morphological and spectral studies of a few important systems[J]. Journal of Power Sources, 1995, 54(1): 76-84.
[48] Zhao Liwei, Watanabe I, Doi T, et al. TG-MS analysis of solid electrolyte interphase (SEI) on graphite negative-electrode in lithium-ion batteries[J]. Journal of Power Sources, 2006, 161(2): 1275-1280.
[49] 陈玉红, 唐致远, 卢星河, 等. 锂离子电池爆炸机理研究[J]. 化学进展, 2006, 18(6): 823-831.
Chen Yuhong, Tang Zhiyuan, Lu Xinghe, et al. Research of explosion mechanism of lithium-ion battery[J]. Progress in Chemistry, 2006, 18(6): 823-831.
[50] Yang Hui, Bang H, Amine K, et al. Investigations of the exothermic reactions of natural graphite anode for Li-ion batteries during thermal runaway[J]. Journal of the Electrochemical Society, 2005, 152(1): A73.
[51] Du Pasquier A, Disma F, Bowmer T, et al. Differential scanning calorimetry study of the reactivity of carbon anodes in plastic Li-ion batteries[J]. Journal of the Electrochemical Society, 1998, 145(2): 472-477.
[52] Sloop S E, Kerr J B, Kinoshita K. The role of Li-ion battery electrolyte reactivity in performance decline and self-discharge[J]. Journal of Power Sources, 2003, 119-121: 330-337.
[53] Hong J S, Maleki H, Al Hallaj S, et al. Electrochemical-calorimetric studies of lithium-ion cells[J]. Journal of the Electrochemical Society, 1998, 145(5): 1489-1501.
[54] Maleki H, Deng Guoping, Anani A, et al. Thermal stability studies of Li-ion cells and components[J]. Journal of the Electrochemical Society, 1999, 146(9): 3224-3229.
[55] Wang Qingsong, Sun Jinhua, Yao Xiaolin, et al. Thermal behavior of lithiated graphite with electrolyte in lithium-ion batteries[J]. Journal of the Electrochemical Society, 2006, 153(2): A329.
[56] Gachot G, Grugeon S, Eshetu G G, et al. Thermal behaviour of the lithiated-graphite/electrolyte interface through GC/MS analysis[J]. Electrochimica Acta, 2012, 83: 402-409.
[57] Golubkov A W, Scheikl S, Planteu R, et al. Thermal runaway of commercial 18650 Li-ion batteries with LFP and NCA cathodes - impact of state of charge and overcharge[J]. RSC Advances, 2015, 5(70): 57171-57186.
[58] Lyu Peizhao, Liu Xinjian, Qu Jie, et al. Recent advances of thermal safety of lithium ion battery for energy storage[J]. Energy Storage Materials, 2020, 31: 195-220.
[59] Doughty D H, Roth E P. A general discussion of Li ion battery safety[J]. Electrochemical Society Interface, 2012, 21(2): 37-44.
[60] Biensan P, Simon B, Pérès J P, et al. On safety of lithium-ion cells[J]. Journal of Power Sources, 1999, 81-82: 906-912.
[61] Arai H, Tsuda M, Saito K, et al. Thermal reactions between delithiated lithium nickelate and electrolyte solutions[J]. Journal of the Electrochemical Society, 2002, 149(4): A401-A406.
[62] MacNeil D D, Dahn J R. The reaction of charged cathodes with nonaqueous solvents and electrolytes: Ⅰ. Li0.5CoO2[J]. Journal of the Electrochemical Society, 2001, 148(11): A1205-A1210.
[63] MacNeil D D, Dahn J R. Test of reaction kinetics using both differential scanning and accelerating rate calorimetries as applied to the reaction of LixCoO2 in non-aqueous electrolyte[J]. The Journal of Physical Chemistry A, 2001, 105(18): 4430-4439.
[64] Wang Haiyan, Tang Aidong, Huang Kelong. Oxygen evolution in overcharged LixNi1/3Co1/3Mn1/3O2 electrode and its thermal analysis kinetics[J]. Chinese Journal of Chemistry, 2011, 29(8): 1583-1588.
[65] Bak S M, Hu Enyuan, Zhou Yongning, et al. Structural changes and thermal stability of charged LiNixMnyCozO2 cathode materials studied by combined in situ time-resolved XRD and mass spectroscopy[J]. ACS Applied Materials & Interfaces, 2014, 6(24): 22594-22601.
[66] Lu Zhonghua, MacNeil D D, Dahn J R. Layered Li[NixCo1–2xMnx]O2 cathode materials for lithium-ion batteries[J]. Electrochemical and Solid-State Letters, 2001, 4(12): A200-A203.
[67] MacNeil D D, Lu Z, Dahn J R. Structure and electrochemistry of Li[NixCo1–2xMnx]O2 (0≤x≤1/2)[J]. Journal of the Electrochemical Society, 2002, 149(10): A1332-A1336.
[68] MacNeil D D, Dahn J R. The reaction of charged cathodes with nonaqueous solvents and electrolytes: Ⅱ. LiMn2O4 charged to 4.2 V[J]. Journal of the Electrochemical Society, 2001, 148(11): A1211-A1215.
[69] Zhang Z, Fouchard D, Rea J R. Differential scanning calorimetry material studies: implications for the safety of lithium-ion cells[J]. Journal of Power Sources, 1998, 70(1): 16-20.
[70] Jiang J, Dahn J R. ARC studies of the thermal stability of three different cathode materials: LiCoO2; Li[Ni0.1Co0.8Mn0.1]O2; and LiFePO4, in LiPF6 and LiBoB EC/DEC electrolytes[J]. Electrochemistry Communications, 2004, 6(1): 39-43.
[71] Martha S K, Haik O, Zinigrad E, et al. On the thermal stability of olivine cathode materials for lithium-ion batteries[J]. Journal of the Electrochemical Society, 2011, 158(10): A1115.
[72] Li Zhihua, Zhang Duanming, Yang Fengxia. Developments of lithium-ion batteries and challenges of LiFePO4 as one promising cathode material[J]. Journal of Materials Science, 2009, 44(10): 2435-2443.
[73] Röder P, Baba N, Friedrich K A, et al. Impact of delithiated Li0FePO4 on the decomposition of LiPF6-based electrolyte studied by accelerating rate calorimetry[J]. Journal of Power Sources, 2013, 236: 151-157.
[74] Kim J, Park K Y, Park I, et al. Thermal stability of Fe-Mn binary olivine cathodes for Li rechargeable batteries[J]. Journal of Materials Chemistry, 2012, 22(24): 11964-11970.
[75] Kalhoff J, Eshetu G G, Bresser D, et al. Safer electrolytes for lithium-ion batteries: state of the art and perspectives[J]. ChemSusChem, 2015, 8(13): 2154-2175.
[76] Gnanaraj J S, Zinigrad E, Asraf L, et al. A detailed investigation of the thermal reactions of LiPF6 solution in organic carbonates using ARC and DSC[J]. Journal of the Electrochemical Society, 2003, 150(11): A1533-A1537.
[77] Zinigrad E, Larush-Asraf L, Gnanaraj J S, et al. Calorimetric studies of the thermal stability of electrolyte solutions based on alkyl carbonates and the effect of the contact with lithium[J]. Journal of Power Sources, 2005, 146(1-2): 176-179.
[78] Park Y U, Seo D H, Kim B, et al. Tailoring a fluorophosphate as a novel 4 V cathode for lithium-ion batteries[J]. Scientific Reports, 2012, 2(1): 1-7.
[79] Ortiz G F, López M C, Li Yixiao, et al. Enhancing the energy density of safer Li-ion batteries by combining high-voltage lithium cobalt fluorophosphate cathodes and nanostructured titania anodes[J]. Scientific Reports, 2016, 6(1): 1-8.
[80] Lebedeva N P, Boon-Brett L. Considerations on the chemical toxicity of contemporary Li-ion battery electrolytes and their components[J]. Journal of the Electrochemical Society, 2016, 163(6): A821-A830.
[81] Ng B, Coman P T, Faegh E, et al. Low-temperature lithium plating/corrosion hazard in lithium-ion batteries: electrode rippling, variable states of charge, and thermal and nonthermal runaway[J]. ACS Applied Energy Materials, 2020, 3(4): 3653-3664.
[82] Nakajima T, Groult H. Fluorinated materials for energy conversion[M]. Amsterdam: Elsevier, 2005.
[83] Terborg L, Weber S, Blaske F, et al. Investigation of thermal aging and hydrolysis mechanisms incommercial lithium ion battery electrolyte[J]. Journal of Power Sources, 2013, 242: 832-837.
[84] Gachot G, Ribière P, Mathiron D, et al. Gas chromatography/mass spectrometry as a suitable tool for the Li-ion battery electrolyte degradation mechanisms study[J]. Analytical Chemistry, 2011, 83(2): 478-485.
[85] Golubkov A W, Fuchs D, Wagner J, et al. Thermal-runaway experiments on consumer Li-ion batteries with metal-oxide and olivin-type cathodes[J]. RSC Advances, 2014, 4(7): 3633-3642.
[86] Roth E P, Orendorff C J. How electrolytes influence battery safety[J]. The Electrochemical Society Interface, 2012, 21(2): 45-49.
[87] Dahn J R, Fuller E W, Obrovac M, et al. Thermal stability of LixCoO2, LixNiO2 and λ-MnO2 and consequences for the safety of Li-ion cells[J]. Solid State Ionics, 1994, 69(3-4): 265-270.
[88] Li J, Zhang Z R, Guo X J, et al. The studies on structural and thermal properties of delithiated LixNi1/3Co1/3Mn1/3O2 (0<x≤1) as a cathode material in lithium ion batteries[J]. Solid State Ionics, 2006, 177(17/18): 1509-1516.
[89] Yang Hui, Shen Xiaodong. Dynamic TGA-FTIR studies on the thermal stability of lithium/graphite with electrolyte in lithium-ion cell[J]. Journal of Power Sources, 2007, 167(2): 515-519.
[90] Kawamura T, Kimura A, Egashira M, et al. Thermal stability of alkyl carbonate mixed-solvent electrolytes for lithium ion cells[J]. Journal of Power Sources, 2002, 104(2): 260-264.
[91] Wang Qingsong, Sun Jinhua, Yao Xiaolin, et al. Thermal stability of LiPF6/EC+DEC electrolyte with charged electrodes for lithium ion batteries[J]. Thermochimica Acta, 2005, 437(1-2): 12-16.
[92] Gnanaraj J S, Zinigrad E, Asraf L, et al. The use of accelerating rate calorimetry (ARC) for the study of the thermal reactions of Li-ion battery electrolyte solutions[J]. Journal of Power Sources, 2003, 119-121: 794-798.
[93] Moshkovich M, Cojocaru M, Gottlieb H E, et al. The study of the anodic stability of alkyl carbonate solutions by in situ FTIR spectroscopy, EQCM, NMR and MS[J]. Journal of Electroanalytical Chemistry, 2001, 497(1-2): 84-96.
[94] Haik O, Ganin S, Gershinsky G, et al. On the thermal behavior of lithium intercalated graphites[J]. Journal of the Electrochemical Society, 2011, 158(8): A913.
[95] Spotnitz R, Franklin J. Abuse behavior of high-power, lithium-ion cells[J]. Journal of Power Sources, 2003, 113(1): 81-100.
[96] Roth E P, Doughty D H, Franklin J. DSC investigation of exothermic reactions occurring at elevated temperatures in lithium-ion anodes containing PVDF-based binders[J]. Journal of Power Sources, 2004, 134(2): 222-234.
[97] Yang Hui, Zhuang G V, Ross P N. Thermal stability of LiPF6 salt and Li-ion battery electrolytes containing LiPF6[J]. Journal of Power Sources, 2006, 161(1): 573-579.
[98] Larsson F, Andersson P, Blomqvist P, et al. Characteristics of lithium-ion batteries during fire tests[J]. Journal of Power Sources, 2014, 271: 414-420.
[99] Ribière P, Grugeon S, Morcrette M, et al. Investigation on the fire-induced hazards of Li-ion battery cells by fire calorimetry[J]. Energy & Environmental Science, 2012, 5(1): 5271-5280.
[100] Larsson F, Bertilsson S, Furlani M, et al. Gas explosions and thermal runaways during external heating abuse of commercial lithium-ion graphite-LiCoO2 cells at different levels of ageing[J]. Journal of Power Sources, 2018, 373: 220-231.
[101] Fu Yangyang, Lu Song, Li Kaiyuan, et al. An experimental study on burning behaviors of 18650 lithium ion batteries using a cone calorimeter[J]. Journal of Power Sources, 2015, 273: 216-222.
[102] Shin J S, Han C H, Jung U H, et al. Effect of Li2CO3 additive on gas generation in lithium-ion batteries[J]. Journal of Power Sources, 2002, 109(1): 47-52.
[103] Li Wen, Li Xing, Chen Mianzhong, et al. AlF3 modification to suppress the gas generation of Li4Ti5O12 anode battery[J]. Electrochimica Acta, 2014, 139: 104-110.
[104] Wu Kai, Yang Jun, Liu Yang, et al. Investigation on gas generation of Li4Ti5O12/LiNi1/3Co1/3Mn1/3O2 cells at elevated temperature[J]. Journal of Power Sources, 2013, 237: 285-290.
[105] Koch S, Birke K, Kuhn R. Fast thermal runaway detection for lithium-ion cells in large scale traction batteries[J]. Batteries, 2018, 4(2): 16.
[106] Essl C, Golubkov A W, Fuchs A. Comparing different thermal runaway triggers for two automotive lithium-ion battery cell types[J]. Journal of the Electrochemical Society, 2020, 167(13): 130542.
[107] Larsson F, Mellander B E. Abuse by external heating, overcharge and short circuiting of commercial lithium-ion battery cells[J]. Journal of the Electrochemical Society, 2014, 161(10): A1611-A1617.
[108] Chen Mingyi, Zhou Dechuang, Chen Xiao, et al. Investigation on the thermal hazards of 18650 lithium ion batteries by fire calorimeter[J]. Journal of Thermal Analysis and Calorimetry, 2015, 122(2): 755-763.
[109] Said A O, Lee C, Stoliarov S I. Experimental investigation of cascading failure in 18650 lithium ion cell arrays: impact of cathode chemistry[J]. Journal of Power Sources, 2020, 446: 227347.
[110] Andersson P, Blomqvist P, Lorén A, et al. Using Fourier transform infrared spectroscopy to determine toxic gases in fires with lithium-ion batteries[J]. Fire and Materials, 2016, 40(8): 999-1015.
[111] Koch S, Fill A, Birke K P. Comprehensive gas analysis on large scale automotive lithium-ion cells in thermal runaway[J]. Journal of Power Sources, 2018, 398: 106-112.
[112] Larsson F. Assessment of safety characteristics for Li-ion battery cells by abuse testing[D]. Gothenburg: Chalmers Tekniska Hogskola, 2014.
[113] Pfrang A, Kriston A, Ruiz V, et al. Safety of rechargeable energy storage systems with a focus on Li-ion technology[M]//Rodriguez-Martinez L M, Omar N. Emerging Nanotechnologies in Rechargeable Energy Storage Systems. Amsterdam: Elsevier, 2017: 253-290.
[114] Huang Lüwei, Zhang Zhaosheng, Wang Zhenpo, et al. Thermal runaway behavior during overcharge for large-format lithium-ion batteries with different packaging patterns[J]. Journal of Energy Storage, 2019, 25: 100811.
[115] Lammer M, Königseder A, Hacker V. Holistic methodology for characterisation of the thermally induced failure of commercially available 18650 lithium ion cells[J]. RSC Advances, 2017, 7(39): 24425-24429.
[116] 潘海鸿, 张沫, 王惠民, 等. 基于多影响因素建立锂离子电池充电内阻的动态模型[J]. 电工技术学报, 2021, 36(10): 2199-2206.
Pan Haihong, Zhang Mo, Wang Huimin, et al. Establishing a dynamic model of lithium-ion battery charging internal resistance based on multiple factors[J]. Transactions of China Electrotechnical Society, 2021, 36(10): 2199-2206.
[117] 杨胜杰, 罗冰洋, 王菁, 等. 基于容量增量曲线峰值区间特征参数的锂离子电池健康状态估算[J]. 电工技术学报, 2021, 36(11): 2277-2287.
Yang Shengjie, Luo Bingyang, Wang Jing, et al. State of health estimation for lithium-ion batteries based on peak region feature parameters of incremental capacity curve[J]. Transactions of China Electrotechnical Society, 2021, 36(11): 2277-2287.
[118] Liao Zhenghai, Zhang Shen, Li Kang, et al. Hazard analysis of thermally abused lithium-ion batteries at different state of charges[J]. Journal of Energy Storage, 2020, 27: 101065.
[119] Somandepalli V, Marr K, Horn Q. Quantification of combustion hazards of thermal runaway failures in lithium-ion batteries[J]. SAE International Journal of Alternative Powertrains, 2014, 3(1): 98-104.
[120] Jiang Fengwei, Liu Kai, Wang Zhirong, et al. Theoretical analysis of lithium-ion battery failure characteristics under different states of charge[J]. Fire and Materials, 2018, 42(6): 680-686.
[121] Stenzel Y P, Börner M, Preibisch Y, et al. Thermal profiling of lithium ion battery electrodes at different states of charge and aging conditions[J]. Journal of Power Sources, 2019, 433: 226709.
[122] Essl C, Golubkov A W, Gasser E, et al. Comprehensive hazard analysis of failing automotive lithium-ion batteries in overtemperature experiments[J]. Batteries, 2020, 6(2): 30.
[123] Zhong Guobin, Mao Binbin, Wang Chao, et al. Thermal runaway and fire behavior investigation of lithium ion batteries using modified cone calorimeter[J]. Journal of Thermal Analysis and Calorimetry, 2019, 135(5): 2879-2889.
[124] Zhang Qingsong, Niu Jianghao, Zhao Ziheng, et al. Research on the effect of thermal runaway gas components and explosion limits of lithium-ion batteries under different charge states[J]. Journal of Energy Storage, 2022, 45: 103759.
[125] Sun Jie, Li Jigang, Zhou Tian, et al. Toxicity, a serious concern of thermal runaway from commercial Li-ion battery[J]. Nano Energy, 2016, 27: 313-319.
[126] Lecocq A, Eshetu G G, Grugeon S, et al. Scenario-based prediction of Li-ion batteries fire-induced toxicity[J]. Journal of Power Sources, 2016, 316: 197-206.
[127] Scheirs J, Kaminsky W. Feedstock recycling and pyrolysis of waste plastics: converting waste plastics into diesel and other fuels[M]. Chichester: John Wiley & Sons, 2006.
[128] Wagner M W, Liebenow C, Besenhard J O. Effect of polysulfide-containing electrolyte on the film formation of the negative electrode[J]. Journal of Power Sources, 1997, 68(2): 328-332.
[129] Wrodnigg G H, Besenhard J O, Winter M. Cyclic and acyclic sulfites: new solvents and electrolyte additives for lithium ion batteries with graphitic anodes?[J]. Journal of Power Sources, 2001, 97-98: 592-594.
[130] Lee J H, Paik U, Hackley V A, et al. Effect of carboxymethyl cellulose on aqueous processing of natural graphite negative electrodes and their electrochemical performance for lithium batteries[J]. Journal of the Electrochemical Society, 2005, 152(9): A1763-A1769.
[131] Ren Dongsheng, Hsu Hengjen, Li Ruihe, et al. A comparative investigation of aging effects on thermal runaway behavior of lithium-ion batteries[J]. eTransportation, 2019, 2: 100034.
[132] 孙丙香, 任鹏博, 陈育哲, 等. 锂离子电池在不同区间下的衰退影响因素分析及任意区间的老化趋势预测[J]. 电工技术学报, 2021, 36(3): 666-674.
Sun Bingxiang, Ren Pengbo, Chen Yuzhe, et al. Analysis of influencing factors of degradation under different interval stress and prediction of aging trend in any interval for lithium-ion battery[J]. Transactions of China Electrotechnical Society, 2021, 36(3): 666-674.
[133] 马泽宇, 姜久春, 张维戈, 等. 锂离子动力电池热老化的路径依赖性研究[J]. 电工技术学报, 2014, 29(5): 221-227.
Ma Zeyu, Jiang Jiuchun, Zhang Weige, et al. Research on path dependence of large format LiMn2O4 battery degradation in thermal aging[J]. Transactions of China Electrotechnical Society, 2014, 29(5): 221-227.
[134] Roth E P, Doughty D H. Thermal abuse performance of high-power 18650 Li-ion cells[J]. Journal of Power Sources, 2004, 128(2): 308-318.
[135] Röder P, Stiaszny B, Ziegler J C, et al. The impact of calendar aging on the thermal stability of a LiMn2O4-Li(Ni1/3Mn1/3Co1/3)O2/graphite lithium-ion cell[J]. Journal of Power Sources, 2014, 268: 315-325.
[136] Li Jia, Zhang Jian, Zhang Xigui, et al. Study of the storage performance of a Li-ion cell at elevated temperature[J]. Electrochimica Acta, 2010, 55(3): 927-934.
[137] Lin Xinrong, Salari M, Arava L M R, et al. High temperature electrical energy storage: advances, challenges, and frontiers[J]. Chemical Society Reviews, 2016, 45(21): 5848-5887.
[138] 贺狄龙, 马冬梅, 段雪琴, 等. 存储条件对磷酸铁锂锂离子电池性能的影响[J]. 电池, 2014, 44(4): 226-228.
He Dilong, Ma Dongmei, Duan Xueqin, et al. Effects of storage condition on the performance of lithium iron phosphate Li-ion battery[J]. Battery Bimonthly, 2014, 44(4): 226-228.
[139] Wu M S, Chiang P C J, Lin J C, et al. Correlation between electrochemical characteristics and thermal stability of advanced lithium-ion batteries in abuse tests—short-circuit tests[J]. Electrochimica Acta, 2004, 49(11): 1803-1812.
[140] Fleischhammer M, Waldmann T, Bisle G, et al. Interaction of cyclic ageing at high-rate and low temperatures and safety in lithium-ion batteries[J]. Journal of Power Sources, 2015, 274: 432-439.
[141] Friesen A, Horsthemke F, Mönnighoff X, et al. Impact of cycling at low temperatures on the safety behavior of 18650-type lithium ion cells: combined study of mechanical and thermal abuse testing accompanied by post-mortem analysis[J]. Journal of Power Sources, 2016, 334: 1-11.
[142] Jeevarajan J. Safety of commercial lithium-ion cells and batteries[M]//Pistoia G. Lithium-Ion Batteries. Amsterdam: Elsevier, 2014: 387-407.
[143] Fathi R, Burns J C, Stevens D A, et al. Ultra high-precision studies of degradation mechanisms in aged LiCoO2/graphite Li-ion cells[J]. Journal of the Electrochemical Society, 2014, 161(10): A1572-A1579.
[144] Zhang Jianbo, Su Laisuo, Li Zhe, et al. The evolution of lithium-ion cell thermal safety with aging examined in a battery testing calorimeter[J]. Batteries, 2016, 2(2): 12.
[145] Essl C, Golubkov A W, Fuchs A. Influence of aging on the failing behavior of automotive lithium-ion batteries[J]. Batteries, 2021, 7(2): 23.
[146] Abd-El-Latif A A, Sichler P, Kasper M, et al. Insights into thermal runaway of Li-ion cells by accelerating rate calorimetry coupled with external sensors and online gas analysis[J]. Batteries & Supercaps, 2021, 4(7): 1135-1144.
[147] Juarez-Robles D, Azam S, Jeevarajan J A, et al. Degradation-safety analytics in lithium-ion cells and modules part Ⅱ. Overcharge and external short circuit scenarios[J]. Journal of the Electrochemical Society, 2021, 168(5): 050535.
[148] Coman P T, Rayman S, White R E. A lumped model of venting during thermal runaway in a cylindrical Lithium Cobalt Oxide lithium-ion cell[J]. Journal of Power Sources, 2016, 307: 56-62.
[149] Coman P T, Mátéfi-Tempfli S, Veje C T, et al. Modeling vaporization, gas generation and venting in Li-ion battery cells with a dimethyl carbonate electrolyte[J]. Journal of the Electrochemical Society, 2017, 164(9): A1858-A1865.
[150] Kim H K, Yang D C, Jang I S, et al. Effects of pretreatment of LM-Ni3.9Co0.6Mn0.3Al0.2 alloy powders in a KOH/NaBH4 solution on the electrode characteristics and inner pressure of nickel-metal-hydride secondary batteries[J]. International Journal of Hydrogen Energy, 2009, 34(23): 9570-9575.
[151] Lu T Y, Chiang C C, Wu S H, et al. Thermal hazard evaluations of 18650 lithium-ion batteries by an adiabatic calorimeter[J]. Journal of Thermal Analysis and Calorimetry, 2013, 114(3): 1083-1088.
[152] Jhu C Y, Wang Y W, Shu C M, et al. Thermal explosion hazards on 18650 lithium ion batteries with a VSP2 adiabatic calorimeter[J]. Journal of Hazardous Materials, 2011, 192(1): 99-107.
[153] Jhu C Y, Wang Y W, Wen C Y, et al. Thermal runaway potential of LiCoO2 and Li(Ni1/3Co1/3Mn1/3)O2 batteries determined with adiabatic calorimetry methodology[J]. Applied Energy, 2012, 100: 127-131.
[154] Zhao Chunpeng, Sun Jinhua, Wang Qingsong. Thermal runaway hazards investigation on 18650 lithium-ion battery using extended volume accelerating rate calorimeter[J]. Journal of Energy Storage, 2020, 28: 101232.
[155] Zhao Chunpeng, Wang Tinghua, Huang Zheng, et al. Experimental study on thermal runaway of fully charged and overcharged lithium-ion batteries under adiabatic and side-heating test[J]. Journal of Energy Storage, 2021, 38: 102519.
[156] Dubaniewicz T H, Barone T L, Brown C B, et al. Comparison of thermal runaway pressures within sealed enclosures for nickel manganese cobalt and iron phosphate cathode lithium-ion cells[J]. Journal of Loss Prevention in the Process Industries, 2022, 76: 104739.
[157] Chen W C, Wang Y W, Shu C M. Adiabatic calorimetry test of the reaction kinetics and self-heating model for 18650 Li-ion cells in various states of charge[J]. Journal of Power Sources, 2016, 318: 200-209.
[158] Lei Boxia, Zhao Wenjiao, Ziebert C, et al. Experimental analysis of thermal runaway in 18650 cylindrical Li-ion cells using an accelerating rate calorimeter[J]. Batteries, 2017, 3(4): 14.
[159] Qin Peng, Sun Jinhua, Wang Qingsong. A new method to explore thermal and venting behavior of lithium-ion battery thermal runaway[J]. Journal of Power Sources, 2021, 486: 229357.
[160] Ostanek J K, Li Weisi, Mukherjee P P, et al. Simulating onset and evolution of thermal runaway in Li-ion cells using a coupled thermal and venting model[J]. Applied Energy, 2020, 268: 114972.
[161] Kim J, Mallarapu A, Finegan D P, et al. Modeling cell venting and gas-phase reactions in 18650 lithium ion batteries during thermal runaway[J]. Journal of Power Sources, 2021, 489: 229496.
[162] Bugryniec P J, Davidson D J N, Brown D S F. Advanced abuse modelling of Li-ion cells-a novel description of cell pressurisation and simmering reactions[J]. Journal of Power Sources, 2020, 474: 228396.
[163] Kong Depeng, Wang Gongquan, Ping Ping, et al. A coupled conjugate heat transfer and CFD model for the thermal runaway evolution and jet fire of 18650 lithium-ion battery under thermal abuse[J]. eTransportation, 2022, 12: 100157.
[164] Dubaniewicz T H Jr, DuCarme J P. Are lithium ion cells intrinsically safe?[J]. IEEE Transactions on Industry Applications, 2013, 49(6): 2451-2460.
[165] Dubaniewicz T H, Zlochower I, Barone T, et al. Thermal runaway pressures of iron phosphate lithium-ion cells as a function of free space within sealed enclosures[J]. Mining, Metallurgy & Exploration, 2021, 38(1): 539-547.
[166] Wen C Y, Jhu C Y, Wang Y W, et al. Thermal runaway features of 18650 lithium-ion batteries for LiFePO4 cathode material by DSC and VSP2[J]. Journal of Thermal Analysis and Calorimetry, 2012, 109(3): 1297-1302.
[167] Wang Congjie, Zhu Yanli, Gao Fei, et al. Thermal runaway behavior and features of LiFePO4/graphite aged batteries under overcharge[J]. International Journal of Energy Research, 2020, 44(7): 5477-5487.
[168] Duh Y S, Theng J H, Chen C C, et al. Comparative study on thermal runaway of commercial 14500, 18650 and 26650 LiFePO4 batteries used in electric vehicles[J]. Journal of Energy Storage, 2020, 31: 101580.
[169] Hatchard T D, MacNeil D D, Basu A, et al. Thermal model of cylindrical and prismatic lithium-ion cells[J]. Journal of the Electrochemical Society, 2001, 148(7): A755.
[170] Li Weifeng, Wang Hewu, Zhang Yajun, et al. Flammability characteristics of the battery vent gas: a case of NCA and LFP lithium-ion batteries during external heating abuse[J]. Journal of Energy Storage, 2019, 24: 100775.
[171] Zhang Yajun, Wang Hewu, Li Weifeng, et al. Quantitative identification of emissions from abused prismatic Ni-rich lithium-ion batteries[J]. eTransportation, 2019, 2: 100031.
[172] 王贺武, 张亚军, 李成, 等. 锂离子动力电池中等荷电状态下热失控产物喷发过程[J]. 储能科学与技术, 2019, 8(6): 1076-1081.
Wang Hewu, Zhang Yajun, Li Cheng, et al. Venting process of lithium-ion power battery during thermal runaway under medium state of charge[J]. Energy Storage Science and Technology, 2019, 8(6): 1076-1081.
[173] 平平. 锂离子电池热失控与火灾危险性分析及高安全性电池体系研究[D]. 合肥: 中国科学技术大学, 2014.
[174] Mier F A, Hargather M J, Ferreira S R. Experimental quantification of vent mechanism flow parameters in 18650 format lithium ion batteries[J]. Journal of Fluids Engineering, 2019, 141(6): 061403.
[175] Mier F A, Hill S M M, Lamb J, et al. Non-invasive internal pressure measurement of 18650 format lithium ion batteries during thermal runaway[J]. Journal of Energy Storage, 2022, 51: 104322.
[176] Jhu C Y, Wang Y W, Wen C Y, et al. Self-reactive rating of thermal runaway hazards on 18650 lithium-ion batteries[J]. Journal of Thermal Analysis and Calorimetry, 2011, 106(1): 159-163.
[177] Mao Binbin, Huang Peifeng, Chen Haodong, et al. Self-heating reaction and thermal runaway criticality of the lithium ion battery[J]. International Journal of Heat and Mass Transfer, 2020, 149: 119178.
[178] Mao Binbin, Zhao Chunpeng, Chen Haodong, et al. Experimental and modeling analysis of jet flow and fire dynamics of 18650-type lithium-ion battery[J]. Applied Energy, 2021, 281: 116054.
[179] Mishra D, Shah K, Jain A. Investigation of the impact of flow of vented gas on propagation of thermal runaway in a Li-ion battery pack[J]. Journal of the Electrochemical Society, 2021, 168(6): 060555.
[180] Lecocq A, Bertana M, Truchot B, et al. Comparison of the fire consequences of an electric vehicle and an internal combustion engine vehicle[C]//Conference Proceedings of Fires in Vehicles, Chicago, USA, 2012: 183-194.
[181] Yuan Liming, Dubaniewicz T, Zlochower I, et al. Experimental study on thermal runaway and vented gases of lithium-ion cells[J]. Process Safety and Environmental Protection, 2020, 144: 186-192.
[182] 黄峥, 秦鹏, 石晗, 等. 过热条件下86 Ah磷酸铁锂电池热失控行为研究[J]. 高电压技术, 2022, 48(3): 1185-1191.
Huang Zheng, Qin Peng, Shi Han, et al. Study on thermal runaway behavior of 86 Ah lithium iron phosphate battery under overheat condition[J]. High Voltage Engineering, 2022, 48(3): 1185-1191.
[183] Galushkin N E, Yazvinskaya N N, Galushkin D N. Mechanism of thermal runaway in lithium-ion cells[J]. Journal of the Electrochemical Society, 2018, 165(7): A1303-A1308.
[184] Wenger M, Waller R, Lorentz V R H, et al. Investigation of gas sensing in large lithium-ion battery systems for early fault detection and safety improvement[C]//IECON 2014 - 40th Annual Conference of the IEEE Industrial Electronics Society, Dallas, TX, USA, 2014: 5654-5659.
[185] Cummings S R, Swartz S L, Frank N B, et al. Systems and methods for monitoring for a gas analyte: US20180003685[P]. 2018-01-04.
[186] 杨启帆, 马宏忠, 刘宝稳, 等. 锂离子电池气体故障特性分析及诊断方法[J]. 高电压技术, 2021, 47(9): 3315-3330.
Yang Qifan, Ma Hongzhong, Liu Baowen, et al. Gas fault characteristics analysis and diagnosis method of lithium-ion battery[J]. High Voltage Engineering, 2021, 47(9): 3315-3330.
[187] Lu Yang, Zhang Shiqi, Dai Shilei, et al. Ultrasensitive detection of electrolyte leakage from lithium-ion batteries by ionically conductive metal-organic frameworks[J]. Matter, 2020, 3(3): 904-919.
[188] Cai Ting, Stefanopoulou A G, Siegel J B. Early detection for Li-ion batteries thermal runaway based on gas sensing[J]. ECS Transactions, 2019, 89(1): 85-97.
[189] 王志荣, 杨赟, 佟轩, 等. 基于气体监测的锂离子电池组热失控自动报警器及其监测方法: CN108008083A[P]. 2018-05-08.
[190] Raghavan A, Kiesel P, Lochbaum A, et al. Battery management based on internal optical sensing: US009553465B2[P]. 2017-01-24.
[191] Hill D, Gully B, Agarwal A, et al. Detection of off gassing from Li-ion batteries[C]//2013 IEEE Energytech, Cleveland, OH, USA, 2013: 1-7.
[192] 王铭民, 孙磊, 郭鹏宇, 等. 基于气体在线监测的磷酸铁锂储能电池模组过充热失控特性[J]. 高电压技术, 2021, 47(1): 279-286.
Wang Mingmin, Sun Lei, Guo Pengyu, et al. Overcharge and thermal runaway characteristics of lithium iron phosphate energy storage battery modules based on gas online monitoring[J]. High Voltage Engineering, 2021, 47(1): 279-286.
[193] 邓孝元. 电动汽车三元锂电池安全监测系统研究[D]. 西安: 长安大学, 2020.
[194] 王春力, 贡丽妙, 亢平, 等. 锂离子电池储能电站早期预警系统研究[J]. 储能科学与技术, 2018, 7(6): 1152-1158. Wang Chunli, Gong Limiao, Kang Ping, et al. Research on early warning system of lithium ion battery energy storage power station[J]. Energy Storage Science and Technology, 2018, 7(6): 1152-1158.
[195] Wang Qian, Zhang Jian, Liu Wei, et al. Quantitative investigation of the gassing behavior in cylindrical Li4Ti5O12 batteries[J]. Journal of Power Sources, 2017, 343: 564-570.
Abstract Li-ion batteries are widely used in electric vehicles, portable electronic devices, and distributed energy storage systems due to their high energy density, long cycle life, low self-discharge rate, and eco-friendly. However, there are still potential safety hazards. It may lead to failure or even fire and explosion accidents when cells are subjected to stress and abuse from mechanical, electrical, and thermal perspectives, posing a significant threat to the overall safety of a variety of battery systems. Therefore, studies on the thermal runaway and safety warning of Li-ion batteries have aroused great concern. On the premise of passing the battery manufacturer’s safety tests, a lot of methods for monitoring and detecting thermal runaway events have been developed to enhance the safety and robustness of Li-ion batteries in various application scenarios. Among these, the gas-based thermal runaway early warning mechanism has improved in reliability, accuracy, and response speed compared with the traditional electrical and thermal signals.
Firstly, this paper summarizes the chemical reactions and their onset temperatures during the thermal runaway of Li-ion batteries, as well as the gases released. The decomposition of the solid electrolyte interphase membrane, the reactions between the active materials, and the decomposition of the electrolyte, especially the intercalated lithium with solvents, can release large amounts of combustible gases including H2, CO, CO2, and hydrocarbons. Flammable gases and solvent vapors are a potential fire hazard when mixed with oxygen. However, Li-ion batteries in various states may exhibit different characteristics of thermal runaway gas production. Thus, the effects and laws of the triggering method, cathode material, cell type, state of charge, and state of health on the components, content, and total amount of gas produced during thermal runaway were comprehensively compared and analyzed. Then the current status of research on the temperature-pressure evolution characteristics during thermal runaway of Li-ion batteries is reviewed from experimental and simulation perspectives, respectively, and the conclusions and limitations of existing studies are summarized. Experimental studies of Li-ion battery eruptions are generally conducted based on sealed chambers, and the output parameters are usually cell temperature, the pressure inside the pressure tank, and gas production components. But these studies are difficult to determine the gas temperature. Therefore, some researchers have simulated gas generation, pressure buildup, gas emission, and combustion processes based on gas production kinetic models. Finally, the current early warning schemes based on gas composition and internal pressure are summarized, and the shortcomings of existing research and potential research directions are discussed to further enhance the safety and robustness of Li-ion battery systems. Early warning methods based on gases (such as H2, CO, electrolyte vapor, etc.) can provide effective early warning for thermal runaway of Li-ion batteries. However, the early warning techniques of a single sensor may not meet the detection requirements of practical engineering applications. The fusion mechanism based on the co-monitoring of multiple parameters may be more effective and comprehensive for the identification of Li-ion battery faults.
Monitoring the thermal runaway processes with gas sensors has proven to be a more effective method than with voltage or with temperature sensors. In addition, the development of new portable gas sensors, such as the MEMS micro-optical gas sensors, the photoacoustic spectrometers, and the infrared spectrometers, may be beneficial in generating accurate early warning signals for applications involving Li-ion batteries with a potential thermal runaway problem.
Keywords:Li-ion batteries, thermal runaway, vent behavior, temperature-pressure evolution characteristics, early warning
中图分类号:TM911.9
DOI: 10.19595/j.cnki.1000-6753.tces.220832
国家自然科学基金(U2166214, 52106111, 52207170)、中央高校基本科研业务费专项资金和王宽诚教育基金资助项目。
收稿日期 2022-05-15
改稿日期 2022-06-19
杨梦洁 女,1997年生,博士研究生,研究方向为锂离子电池热失控早期预警。E-mail:mjyang19@stu.xjtu.edu.cn
杨爱军 男,1986年生,教授,博士生导师,研究方向为电力设备状态诊断。E-mail:yangaijun@mail.xjtu.edu.cn(通信作者)
(编辑 李冰)