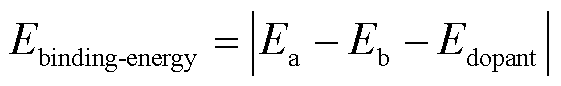 (1)
(1)
摘要 基于密度泛函理论(DFT)的第一性原理计算,研究了SOF2和SO2F2两种SF6分解气体在SnS2及Osn(n=1~2)掺杂改性的SnS2材料表面的气敏响应。从吸附能、能带结构、态密度、前线轨道等方面,对比分析三种材料分别对SOF2及SO2F2的气敏响应机理。研究发现,本征SnS2对SOF2及SO2F2的气敏响应特性不佳,但在Os掺杂后,掺杂处成为材料表面的活性位点,有效地提高了两种气体在SnS2表面的气敏响应特性:Osn-SnS2(n=1~2)对SOF2表现出良好的吸附效果,其中Os2-SnS2对SO2F2也具有理想的传感特性。该研究为实验开发用于检测SF6分解气体的高性能气敏传感器提供了理论基础。
关键词:密度泛函理论 SF6分解气体 Osn-SnS2 吸附特性
六氟化硫(SF6)气体设备由于灭弧能力强、结构紧凑、运行安全、绝缘性能高,被广泛用于超特高压电力系统中[1-4],但是设备在长时间运行中可能存在局部放电[5]、火花放电、电弧放电和局部过热等故障[6-8],导致SF6气体分解。在实际SF6电气设备中,除SF6外,还含有水分、空气、矿物油等杂质[9],SF6分解产物与水和氧气发生化学反应会生成多种有毒、有腐蚀性并且稳定的气体产物,如SO2F2、SOF2、SO2和H2S等,严重降低了SF6气体的绝缘性能[10-12],因此及时发现故障对含SF6气体的电气设备极其重要。由于它的灵敏度高、检测速度快,更不易受现场环境和电磁信号的干扰,故是诊断气体绝缘金属封闭开关(Gas Insulated Switchgear, GIS)设备内部潜伏性故障较有效的一种带电检测方式[13-15]。SOF2和SO2F2是SF6分解组分中的两种主要的典型分解组分(10-6级)[16]。现有的研究表明,气体传感器对SOF2和SO2F2的气敏响应低于其他气体分解产物(SO2,H2S等)[17-19],并且需要多种传感器的配合,才能实现对气体浓度和类型的准确检测,因此有必要寻找一种用于SOF2和SO2F2检测的新型气体传感材料。
二维(2D)材料以其优异的热学、力学和结构性能以及较高的表面积-体积比引起了科学家们极大的兴趣[20- 21],多种2D材料被用于吸附小分子气体:SnO2[22]、石墨烯[23-25]、MoS2[26-29]。本课题组前期研究了Mn掺杂改性后的石墨烯对CO、C2H2、CH4的气敏响应[30],同时研究了Ni掺杂的MoS2单层对H2S以及SO2的气敏响应特性[31]。SnS2与MoS2均可通过机械剥落和化学气相沉积法进行制备[32-33]。近年来,有较多小分子气体在单层SnS2或掺杂改性后的单层SnS2上的气敏响应的研究结果,这些气体包括COx、NOx、NH3等[21, 34],也有SnS2对SOF2和SO2F2的气敏响应性能的研究[35],然而其吸附能较低,考虑通过掺杂,提高SnS2对SOF2和SO2F2的气敏响应。在过渡金属中,锇(Os)作为掺杂物对基底材料进行修饰后,体系的吸附效果会有显著改善。研究表明在众多贵金属中,TiO2(101)表面掺杂Os后对CH4的吸附能最大[36],在2D结构的石墨烯表面掺杂过渡金属后,掺杂Os的石墨烯对CO和H2具有良好的气敏响应特性[37-38]。相关研究表明:通过掺杂过渡金属可以改善SnS2单分子膜的气敏特性[19]。目前尚无Os掺杂于SnS2表面的相关研究,本文使用Os修饰SnS2,研究其对SOF2和SO2F2的气敏响应。
本文基于密度泛函理论(Density Functional Theory, DFT)在Dmol3模块中研究了SOF2和SO2F2气体与本征SnS2材料和Osn-SnS2(n=1, 2)材料表面的相互作用机制[39]。采用广义梯度近似(Generalized Gradient Approximation, GGA)泛函中的PBE处理电子-电子相互作用的交换-关联能以提供固体物质与气体分子之间的准确预测[40]。通过TS方法进行DFT-D校正,在计算过程中考虑自旋并使用对称以加快计算速度,自洽场迭代收敛判据设置为10-6Ha(1Ha=27.212eV)布里渊区采样点(K点)设置为5´5´1[35],使用单个有效势替代内核电子。基组设置采用双数值轨道基组+轨道极化函数。本研究中构造的超晶胞纳米单层是重复性的结构,建立真空层以避免一个单层对另一单层的影响,周期边界条件设置为15Å´15Å´33Å(1Å=10-10m)。
Osn掺杂于SnS2单层表面的结合能Ebinding-energy定义为
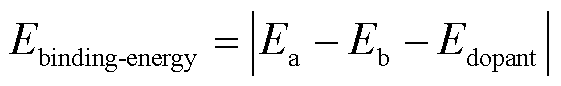 (1)
(1)
式中,Ea为掺杂后的总能量;Eb为本征SnS2的能量;Edopant为Osn的能量。结合能越大,该掺杂改性后的材料越稳定。
SOF2、SO2F2在本征SnS2和Osn-SnS2材料表面的吸附能(Eads)计算公式为
 (2)
(2)
式中,Egas和Esurface分别为孤立的气体和基底的能量;Egas/surface为气体与基底相互作用后的能量。由吸附能的计算公式可知,若吸附能为负,则该反应可以自发地进行,吸附过程中体系放出热量;反之吸收热量。为了研究吸附过程的电荷转移情况,基于Mulliken布居分析计算电荷转移量QT,电荷转移量(单位e)定义为
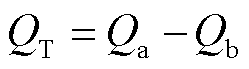 (3)
(3)
式中,Qa为吸附后气体分子的带电量;Qb为吸附前气体分子的带电量。若QT>0,则电子从气体分子转移到基底上。
2.1.1 气体吸附前的结构模型
用前文计算参数中的计算方法构造和优化模型,以结合能最大为判据得到最优结构。图1a为本征SnS2模型,与Sn*所成的六个Sn-S键均为2.611Å。图1b、图1c展示了SOF2和SO2F2的原始结构,键角标注于图中。在SnS2表面分别掺杂一个Os原子和两个Os原子组成团簇,掺杂位点选择空位上方,Sn原子顶部,S原子顶部,Sn与S连线上方,根据式(1),选择结合能最大的结构进行后续研究,具体结合能列于表1中。掺杂一个Os原子的最稳定结构如图1d所示,Os原子处成为活性位点处于Sn顶部。图1d展示了利用Os2团簇修饰本征SnS2后得到的最优结构,Os2团簇处于空位上方,两个Os原子分别位于S原子顶部,Os2团簇附近成为活性位点。优化后结构中的距离参数与结合能见表1。在图1d中,位于Os下方的Sn原子断裂了三个Sn-S键,与Os原子形成一个化学键,Sn-S键的断裂使得S原子存在单电子,能与Os原子成键,形成的三个S-Os键长列于表1中,虚线圈内SnS2结构呈现明显变化,且Os1与单层SnS2的结合能已达到-6.411eV,说明Os1与SnS2的相互作用强烈,结合稳定,其中负号表示该反应放出热量,可以自发进行。Os2团簇与SnS2单层掺杂后的结构如图1e所示,由于Os2团簇远离底部的Sn原子,Os2团簇与Sn原子并未成键,但与三个S原子形成三个化学键,其键长列于表1中,掺杂后的SnS2在结构上也发生改变,与图1a相比,SnS2表面结构变得略微不规则,其结合能为-4.169eV,与SnS2的结合较稳定。
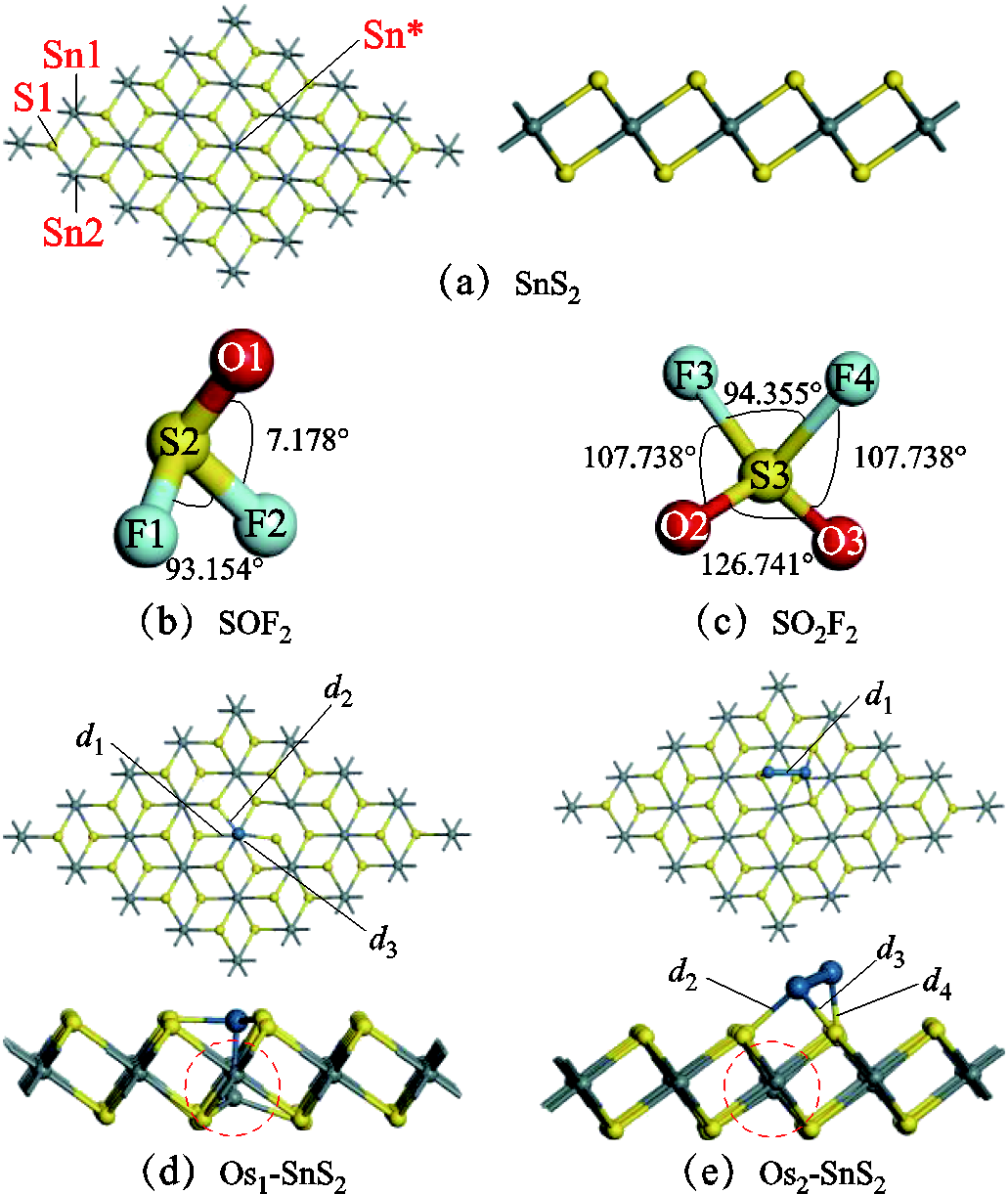
图1 吸附前的基底和吸附前的气体结构
Fig.1 Substrate before adsorption and gas structure before adsorption
表1 气体分子与掺杂Os前后SnS2结构参数与结合能
Tab.1 The structural parameter and binding energy of gas molecules and SnS2 before and after Os doping

结构d/ÅEbinding-energy/eV SnS2S1-Sn1/ S1-Sn2:2.611— SOF2S2-O1: 1.460S2-F1/S2-F1: 1.670— SO2F2S3-F3/S3-F3: 1.611S3-O2/S3-O3: 1.442— Os1-SnS2d1: 2.225d2: 2.232d3: 2.250-6.411 Os2-SnS2d1:2.293d2: 2.280d3: 2.272d4: 2.181-4.169
2.1.2 气体吸附后的结构模型
SOF2与SO2F2吸附后的结构分别如图2、图3所示,吸附距离标注于图中,为了直观比较,表2中汇总了吸附距离。

图2 SOF2吸附后的结构
Fig.2 Structures after SOF2 adsorption

图3 SO2F2吸附后的结构
Fig.3 Structures after SO2F2 adsorption
表2 SOF2及SO2F2的吸附参数
Tab.2 Adsorption parameters of SOF2 and SO2F2
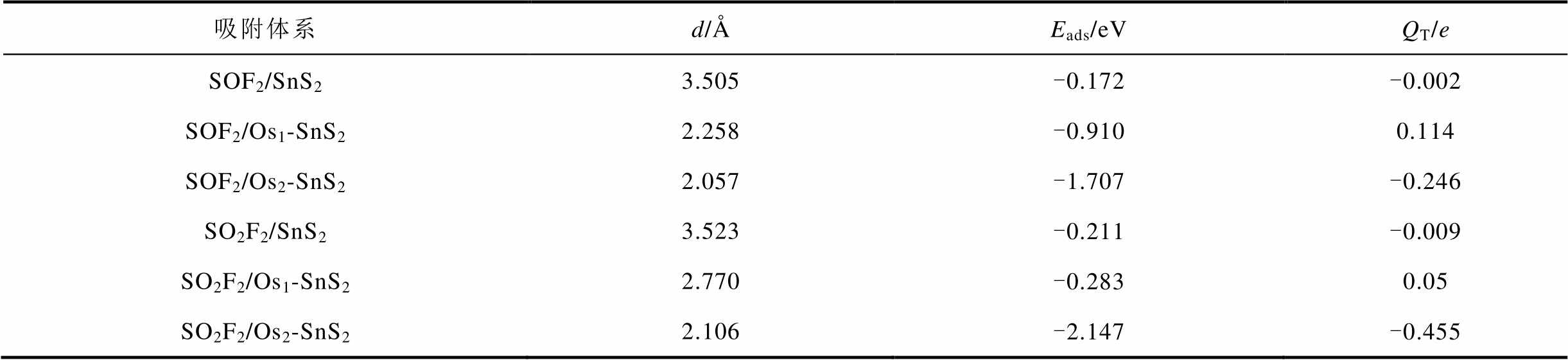
吸附体系d/ÅEads/eVQT/e SOF2/SnS23.505-0.172-0.002 SOF2/Os1-SnS22.258-0.9100.114 SOF2/Os2-SnS22.057-1.707-0.246 SO2F2/SnS23.523-0.211-0.009 SO2F2/Os1-SnS22.770-0.2830.05 SO2F2/Os2-SnS22.106-2.147-0.455
对SOF2的气体吸附情况做如下分析:Osn掺杂前吸附距离为3.505Å,吸附能仅为-0.172eV,与文献[23]中的一致,SOF2中各键角分别为(“()”内表示与原键角的差值):O1-S2-F1:107.356°(-0.362°),O1-S2-F2:107.359°(-0.359°),F1-S2-F2:93.164°(+0.01°)。掺杂Os1后吸附能为-0.910eV,SOF2失去0.114e电子,SOF2各键角分别为:O1-S2-F1:106.354°(-1.364°),O1-S2-F2:106.323°(-1.359°),F1-S2-F2:91.713°(-1.441°),且S与Os成键,可见SOF2与吸附前的结构有较大差异。掺杂Os2团簇后吸附能达到-1.707eV,SOF2的结构改变更加明显,O原子和S原子都与其中一个Os原子成键。SOF2各键角如下:O1-S2-F1:108.400°(+0.682°),O1-S2-F2:94.158°(-13.56°),F1-S2-F2:87.101°(-6.053°),SOF2作为受主,得到0.246e电子。掺杂后吸附距离显著缩短,吸附能提高,从吸附能和吸附距离看,Osn的掺杂有利于SnS2吸附SOF2。
SO2F2的吸附情况可与SOF2做相同分析,与SOF2相比较,吸附后SO2F2距离基底的距离都较大。在掺杂Os1后,SO2F2在Os1-SnS2上的吸附能提高不明显,SO2F2与基底并未成键,Os2团簇的掺杂才使吸附能有显著提高,并且形成了三个新的化学键,分别是一个S-Os键,两个O-Os键。观察SO2F2的结构变化,本征SnS2材料吸附SO2F2后,SO2F2各键角如下:F3-S3-O2:107.573°(-0.165°),F4-S3-O3:107.903°(+0.165°),F3-S3-F4:94.395°(+0.04°),O2-S3-O3:126.683°(-0.058°),吸附前后键角几乎没有改变。掺杂Os1后,SO2F2失去0.05e电子,各键角如下:F3-S3-O2:106.570°(-1.168°),F4-S3-O3:108.595°(+0.857°),F3-S3-F4:94.873°(+0.518°),O2-S3-O3:126.108°(-0.633°),SO2F2气体的结构变化较图3a中大。掺杂Os2团簇后,SO2F2气体的键角改变更加明显:F3-S3-O2:91.652°(-16.086°),F4-S3-O3:98.588°(-9.15°),F3-S3-F4:85.138° (-9.217°),O2-S3-O3:107.273°(-19.468°),SO2F2得到0.455e电子,说明Os2-SnS2与SO2F2的相互作用在三种基底中最强烈。Os的掺杂提高了SnS2对SO2F2的吸附效果。
2.2.1 能带分析
气体吸附前体系的能带如图4所示,掺杂前价带与导带之间的带隙为1.530eV,属于半导体材料,掺杂Os1后,带隙明显减小,电子更容易获得能量跳跃至导带导电,掺杂Os2团簇后带隙几乎为0,可视为金属材料。
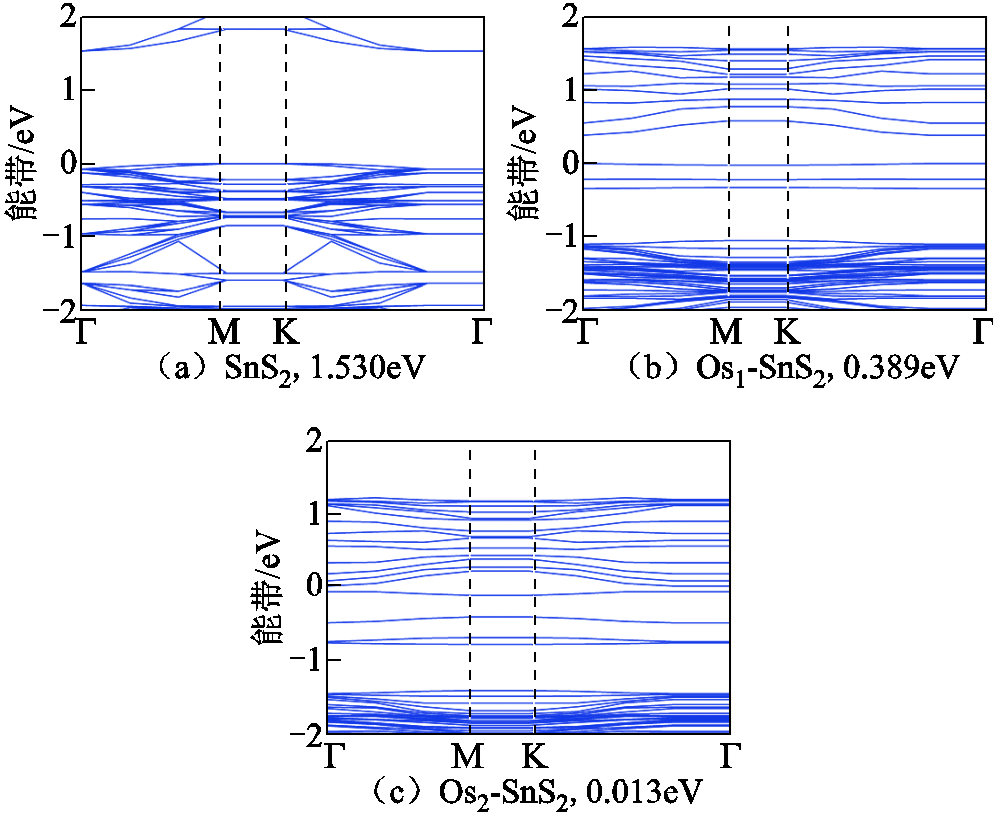
图4 气体吸附前的能带
Fig.4 The band structure before gas adsorption
SOF2及SO2F2吸附后的体系能带如图5所示,其中图5a~图5c是与SOF2相互作用后的体系,图5d~图5f为吸附SO2F2后的体系。
本征SnS2材料吸附SOF2后,杂质能级增多,即使获得的能量较小也可以逐级迁跃至导带导电,且带隙减小,说明吸附后电导率提高。SO2F2吸附后,单层SnS2带隙也减小,SOF2/SnS2减小0.13%(见图5a),SO2F2/SnS2减小0.26%(见图5b),减小程度较小,电导率变化不明显,不适合用作传感器。气体吸附在Os1-SnS2体系后,SOF2与SO2F2所在系统的带隙分别增加了6.68%、2.82%,电导率降低,导电性能下降。两种气体吸附在Os2-SnS2体系后带隙增大,电子迁移至导带导电比吸附前困难,电导率下降。因为Osn-SnS2在吸附前后电导率变化较大,所以Osn-SnS2满足作为检测SOF2和SO2F2传感器的要求。
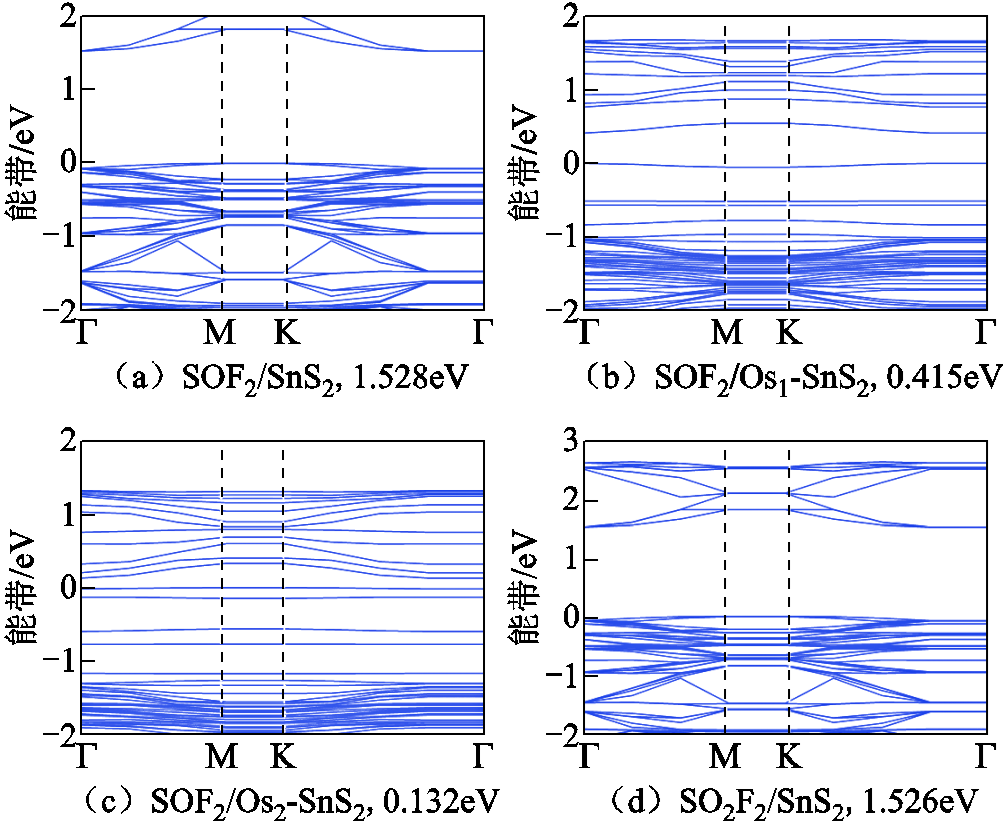

图5 气体吸附后的能带
Fig.5 The band structure after gas adsorption
2.2.2 态密度分析
通过具体分析总态密度(Total Density of States, TDOS)以及分波态密度(Partial Density of State PDOS)在吸附气体前后的变化情况,进一步探究体系吸附前后电导率的变化。图6所示为SOF2吸附前后的总态密度以及吸附后体系的分波态密度,虚线表示费米能级。
在图6a左图中,态密度整体向左偏移,但在向左移动的过程中,费米能级附近态密度有所上升,虽然导带填充的电子数量减少,但电子从价带跃迁到导带的概率提高,电导率提高。从图6a右图可以看出O-2p与Sn-5d轨道在-1eV处存在轨道杂化,同时O-2p、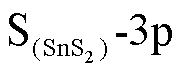 、Sn-5p在-2eV处具有峰值重叠,这些轨道的杂化导致图6a左图两条曲线的差别。在图6b中O-2p、
、Sn-5p在-2eV处具有峰值重叠,这些轨道的杂化导致图6a左图两条曲线的差别。在图6b中O-2p、 、
、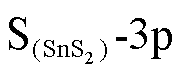 、Os-5d轨道在-1eV、-4.5eV处存在轨道杂化,导致总态密度在SOF2吸附后有略微上移,但在费米能级附近总态密度下压,电导率下降。图6b的情况相似,电子从费米能级左方转移到右方的难度提高,电导率下降,形成这一现象的主要原因是O-2p轨道和Os-5d轨道在-2.5eV处存在峰值重叠,O-2p和S(SnS2)-3p轨道在-6eV存在轨道杂化。
、Os-5d轨道在-1eV、-4.5eV处存在轨道杂化,导致总态密度在SOF2吸附后有略微上移,但在费米能级附近总态密度下压,电导率下降。图6b的情况相似,电子从费米能级左方转移到右方的难度提高,电导率下降,形成这一现象的主要原因是O-2p轨道和Os-5d轨道在-2.5eV处存在峰值重叠,O-2p和S(SnS2)-3p轨道在-6eV存在轨道杂化。
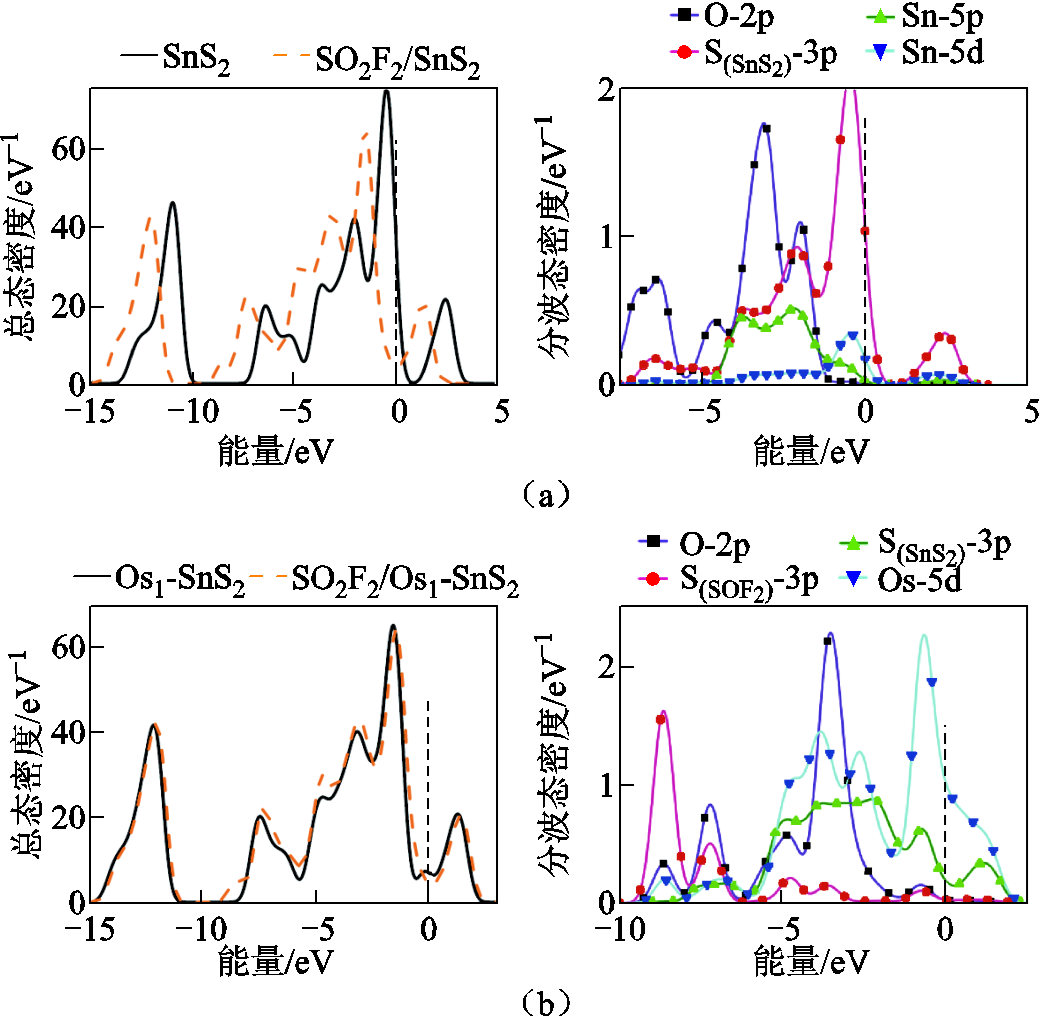

图6 SOF2吸附前后体系的总态密度(TDOS)和吸附后体系的分波态密度(PDOS)
Fig. 6 TDOS of the systems before and after SOF2 adsorption and PDOS after SOF2 adsorption
图7a~图7c是SO2F2气体在SnS2和Osn-SnS2材料表面吸附前后的总态密度,图7d~图7f展示了气体吸附后的部分原子轨道情况。
图7a中费米能级附近的态密度几乎没有改变,结合表2中的吸附能,分别为-0.211eV,-0.283eV,说明吸附程度较弱,吸附对基底的影响较小。图7b中吸附后的总态密度在费米能级附近下降,价带中能够导电的自由电子减少,电子转移困难,结果与能带分析一致。可以由图7b右图解释总态密度变化的原因,图7c右图中O-2p、Os-5d轨道在-6eV处存在杂化,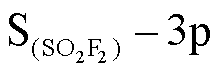 , O-2p, Os-5d在-7eV,-9eV处存在杂化。
, O-2p, Os-5d在-7eV,-9eV处存在杂化。

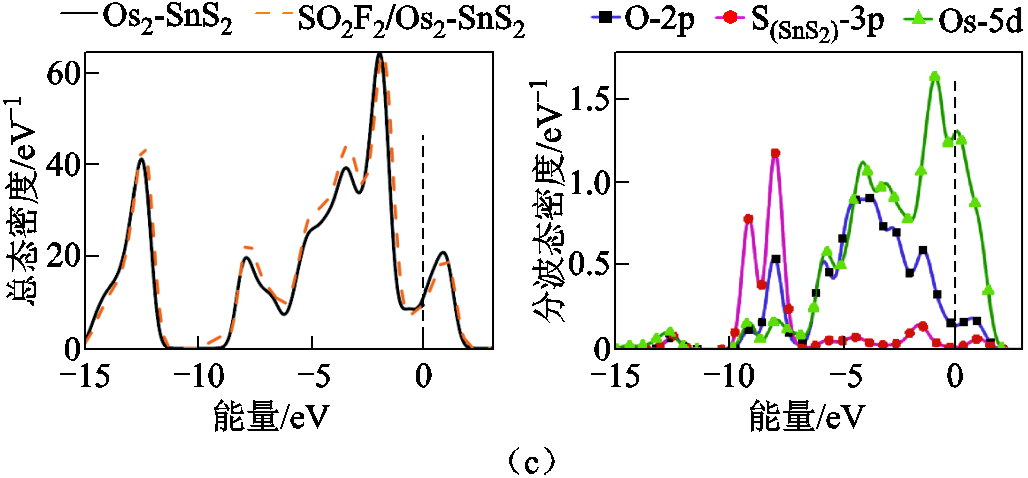
图7 SO2F2吸附前后体系的总态密度(TDOS)和吸附后体系的分波态密度(PDOS)
Fig.7 TDOS of the systems before and after SO2F2 adsorption and PDOS of the systems after SO2F2 adsorption
为了深入分析SOF2与SO2F2在SnS2及Osn-SnS2表面上的反应机制,研究了HOMO-LUMO,其中HOMO指最高占据分子轨道,LUMO指最低未占据分子轨道。图8a是本征SnS2的HOMO-LUMO分布图,EHOMO为-6.851eV,ELUMO为-5.258eV,能隙Eg(Eg=ELUMO-EHOMO)为1.593eV。经过Osn掺杂改性后SnS2的HOMO-LUMO分布如图8b和图8c所示,HOMO-LUMO部分转移到Os原子附近,能隙明显小于图8a,同样说明SnS2是半导体材料,Osn-SnS2属于导体材料。

图8 气体吸附前的前线轨道(HOMO和LUMO分布)
Fig.8 Molecular orbitals before gas adsorption (HOMO and LUMO distribution)
图9与图10分别是SOF2和SO2F2吸附后的HOMO-LUMO分布,与图8对比,吸附后HOMO-LUMO分布有所改变。SOF2与SO2F2吸附在本征SnS2表面后,能隙依次减小0.25%、0.19%,减小程度小,电导率增大效果不明显,与能带和态密度的分析结果一致。Os1-SnS2吸附两种气体后,能隙有不同程度的增大,电导率下降。SOF2吸附在Os2-SnS2表面后,虽然能隙减小,但HOMO、LUMO之间比较分散,电子转移较困难。Os2-SnS2与SO2F2气体分子相互作用后,SO2F2上也存在HOMO-LUMO的分布,SO2F2/Os2-SnS2体系能隙增大,分子不易被激发,电导率下降明显。
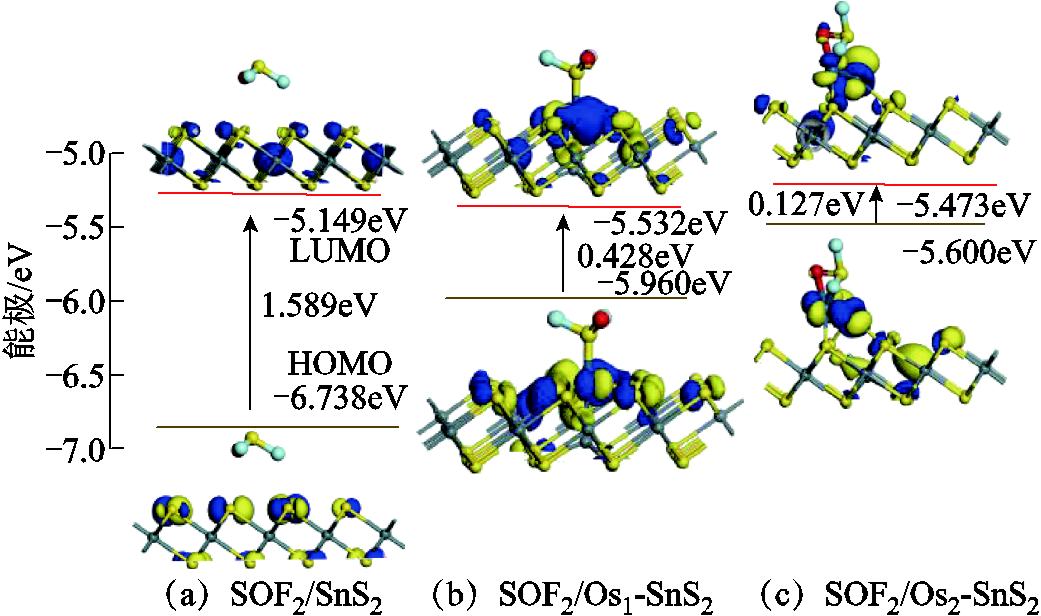
图9 SOF2吸附后的分子轨道(HOMO和LUMO分布)
Fig.9 Molecular orbitals after SOF2 adsorption (HOMO and LUMO distribution)

图10 SO2F2吸附后的分子轨道(HOMO和LUMO分布)
Fig.10 Molecular orbitals after SO2F2 adsorption (HOMO and LUMO distribution)
本文基于密度泛函理论的第一性原理,深入探究了SF6分解气体在本征SnS2以及Osn-SnS2材料表面的吸附机制。通过对体系的结构、能带、态密度和前线轨道等的分析,研究了三种体系分别吸附两种气体前后的电导率的变化。研究发现,本征SnS2吸附两种气体后电导率变大,并且从能带和HOMO-LUMO的变化情况做进一步分析可以得出电导率变化程度较小。Osn-SnS2吸附SOF2和SO2F2后,电导率都变小且变化程度比本征SnS2吸附气体后明显,说明Os的掺杂有效提高了SnS2材料对SOF2和SO2F2气体的灵敏度。由吸附能的强弱判定,在三种基底中,只有Osn-SnS2对SOF2和Os2-SnS2对SO2F2的作用是化学吸附,并对比Osn-SnS2对两种气体的吸附效果,认为Os1-SnS2和Os2-SnS2都可以作为检测SOF2的传感器;而Os2-SnS2对于SO2F2检测更具有前景。此仿真为实验研究Osn-SnS2对SOF2和SO2F2的气敏响应提供了理论基础。
参考文献
[1] 窦小晶, 叶日新, 付纪华, 等. SF6气体分解产物检测技术及其应用的研究现状[J]. 电网与清洁能源, 2019, 35(7): 24-31.
Dou Xiaojing, Ye Rixin, Fu Jihua, et al. Research status of SF6 gas decomposition products detection methods and their application[J]. Power System and Clean Energy, 2019, 35(7): 24-31.
[2] Cao Wenhai, Gui Yingang, Chen Tao, et al. Adsorption and gas-sensing properties of Pt2-GaNNTs for SF6 decomposition products[J]. Applied Surface Science, 2020, 524: 146570.
[3] 张晓星, 王宇非, 崔兆仑, 等. 不同填充材料对介质阻挡放电降解SF6的实验研究[J]. 电工技术学报, 2021, 36(2): 397-406.
Zhang Xiaoxing, Wang Yufei, Cui Zhaolun, et al. Experimental study on the degradation of SF6 by dielectric barrier discharge with different packing materials[J]. Transactions of China Electrotechnical Society, 2021, 36(2): 397-406.
[4] 王宝山, 余小娟, 侯华, 等. 六氟化硫绝缘替代气体的构效关系与分子设计技术现状及发展[J]. 电工技术学报, 2020, 35(1): 21-33.
Wang Baoshan, Yu Xiaojuan, Hou Hua, et al. Review on the developments of structure-activity relationship and molecular design of the replacement dielectric gases for SF6[J]. Transactions of China Electrotechnical Society, 2020, 35(1): 21-33.
[5] 陆云才, 胡汉巧, 蔚超, 等. 基于超声波法的变压器重症监护系统研制及应用[J]. 电力工程技术, 2017, 36(2): 94-98.
Lu Yuncai, Hu Hanqiao, Wei Chao, et al. development and application of transformer intensive care system based on ultrasonic method[J]. Electric Power Engineering Technology, 2017, 36(2): 94-98.
[6] Chu Jifeng, Wang Xiaohua, Wang Dawei, et al. Highly selective detection of sulfur hexafluorride decomposition components H2S and SOF2 employing sensors based on tin oxide modified reduced graphene oxide[J]. Carbon, 2018, 135: 95-103.
[7] Wang Jingxuan, Zhou Qu, Zeng Wen. Competitive adsorption of SF6 decompositions on Ni-doped ZnO (100) surface: computational and experimental study[J]. Applied Surface Science, 2019, 479: 185-197.
[8] 张晓星, 董星辰, 陈秦川. 锐钛矿型(101)晶面吸附SF6局部放电分解组分的气敏机理分析[J]. 电工技术学报, 2017, 32(3): 200-209.
Zhang Xiaoxing, Dong Xingchen, Chen Qinchuan. Gas sensing mechanism analysis of SF6 decomposed gases adsorption on anatase (101) surface under partial discharge[J]. Transactions of China Electrotechnical Society, 2017, 32(3): 200-209.
[9] 季严松, 王承玉, 杨韧, 等. SF6气体分解产物检测技术及其在GIS设备故障诊断中的应用[J]. 高压电器, 2011, 47(2): 100-103, 107.
Ji Yansong, Wang Chengyu, Yang Ren, et al. Measuring technique of SF6 decomposition products and its application to fault diagnosis of GIS[J]. High Voltage Apparatus, 2011, 47(2): 100-103, 107.
[10] Wu Peng, Zhang Xiaoxing, Chen Dachang, et al. Adsorption of SF6 decomposed products on ZnO-modified C3N: a theoretical study[J]. Nanoscale Research Letters, 2020, 15(1): 1-11.
[11] 王邸博, 陈达畅, 皮守苗, 等. 基于密度泛函理论的SF6分解组分在ZnO (0001) 吸附及传感性能研究[J] 电工技术学报, 2020, 35(7): 1592-1602.
Wang Dibo, Chen Dachang, Pi Shoumiao, et al. Density functional theory study of SF6 decomposed products over ZnO(0001) with gas sensing properties[J]. Transactions of China Electrotechnical Society, 2020, 35(7): 1592-1602.
[12] 赵建利, 姚顺, 岳永刚, 等. 500kV SF6瓷质套管多工况仿真与故障分析[J]. 电工技术学报, 2021, 36(S2): 736-745.
Zhao Jianli, Yao Shun, Yue Yonggang, et al. Simulation and failure analysis of 500kV SF6 porcelain bushing under complicated working conditions[J]. Transactions of China Electrotechnical Society, 2021, 36(S2): 736-745.
[13] 陈东, 雷诗铭, 刘春意, 等. 基于SF6气体分解产物检测的潜伏性故障判断[J]. 湖北电力, 2020, 44(3): 70-74.
Chen Dong, Lei Shiming, Liu Chunyi, et al. Latent fault judgment based on SF6 gas decomposition products detection[J]. Hubei Electric Power, 2020, 44(3): 70-74.
[14] 周朕蕊, 韩冬, 赵明月, 等. SF6替代气体分解特性的研究综述[J]. 电工技术学报, 2020, 35(23): 4998-5014.
Zhou Zhenrui, Han Dong, Zhao Mingyue, et al. Review on decomposition characteristics of SF6 alternative gases[J]. Transactions of China Electrotechnical Society, 2020, 35(23): 4998-5014.
[15] 桂银刚,许文龙,张晓星,等. TiO2掺杂石墨烯对 SO2气体的气敏特性研究[J]. 电工技术学报,2021,36(21): 4590-4597.
Gui Yingang, Xu Wenlong, Zhang Xiaoxing, et al. Adsorption property of SO2 gas on TiO2-doped graphene[J]. Transactions of China Electrotechnical Society, 2021, 36(21): 4590-4597.
[16] Qian Hai, Deng Jun, Zhou Haibin. First-principles study of Pd-MoSe2 as sensing material for characteristic SF6 decomposition components[J]. AIP Advances, 2019, 9(12): 125013.
[17] Liu Daikun, Gui Yingang, Ji Chang, et al. Adsorption of SF6 decomposition components over Pd (111): A density functional theory study[J]. Applied Surface Science, 2019, 465: 172-179.
[18] Wang Yao, Gui Yingang, Ji Chang, et al. Adsorption of SF6 decomposition components on Pt-3-TiO2 (101) surface: a DFT study[J]. Applied Surface Science, 2018, 459: 242-248.
[19] Guo Haojie, Zheng Kai, Cui Heping, et al. High sensitivity gas sensor to detect SF6 decomposition components based on monolayer antimonide phosphorus[J]. Chemical Physics Letters, 2020, 756(1): 137868.
[20] Upadhyay D, Roondhe B, Pratap A, et al. Two-dimensional delafossite cobalt oxyhydroxide as a toxic gas sensor[J]. Applied Surface Science, 2019, 476: 198-204.
[21] Wang Xiaodong, Wang Jing. Effects of Pt and Au adsorption on the gas sensing performance of SnS2 monolayers: a DFT study[J]. Materials Science in Semiconductor Processing, 2021, 121: 105416.
[22] Patel A, Roondhe B, Jha P K. Ni doping effect on the electronic and sensing properties of 2D SnO2[C]// International Conference on Nanomaterials for Energy Conversion & Storage Applications, Necsa, 2018, 1961(1): 030039.
[23] Zhang Xiaoxing, Fang Rongxing, Chen Dachang, et al. Using Pd-Doped γ-Graphyne to detect dissolved gases intransformer oil: a density functional theory investigation[J]. Nanomaterials, 2019, 9(10): 9101490.
[24] Zhang Xiaoxing, Yu Lei, Gui Yingang, et al. First-principles study of SF6 decomposed gas adsorbed on Au-decorated graphene[J]. Applied Surface Science, 2016, 367: 259-269.
[25] Zhang Xiaoxing, Yu Lei, Wu Xiaoqing, et al. Experimental sensing and density functional theory study of H2S and SOF2 adsorption on Au-modified graphene[J]. Advanced Science, 2015, 2(11): 612-612.
[26] Fan Yuehua, Zhang Jinyan, Qiu Yuzhi, et al. A DFT study of transition metal (Fe, Co, Ni, Cu, Ag, Au, Rh, Pd, Pt and Ir)-embedded monolayer MoS2 for gas adsorption[J]. Computational Materials Science, 2017, 138: 255-266.
[27] Qian Hai, Lu Wenhao, Wei Xiaoxing, et al. H2S and SO2 adsorption on Pt-MoS2 adsorbent for partial discharge elimination: a DFT study[J]. Results in Physics, 2019, 12 107-112.
[28] Zhu Jia, Zhang Hui, Tong Yawen, et al. First-principles investigations of metal (V, Nb, Ta)-doped monolayer MoS2: Structural stability, electronic properties and adsorption of gas molecules[J]. Applied Surface Science, 2017, 419: 522-530.
[29] Li Tao, Gui Yingang, Zhao Wenhao, et al. Palladium modified MoS2 monolayer for adsorption and scavenging of SF6 decomposition products: A DFT study[J]. Physica E: Low-dimensional Systems and Nanostructures, 2020, 123: 114178.
[30] Gui Yingang, Peng Xiao, Liu Kai, et al. Adsorption of C2H2, CH4 and CO on Mn-doped graphene: atomic, electronic, and gas-sensing properties[J]. Physica E: Low-dimensional Systems and Nanostructures, 2020, 119: 113959.
[31] Wei Huangli, Gui Yingang, Kang Jian, et al. A DFT study on the adsorption of H2S and SO2 on Ni doped MoS2 monolayer[J]. Nanomaterials, 2018, 8(9): 8090646.
[32] Li Bo, Xing Tao, Zhong Mianzeng, et al. A two-dimensional Fe-doped SnS2 magnetic semiconductor[J]. Nature communications, 2017, 8: 1-7.
[33] Xu Liping, Zhang Peng, Jiang Huaning, et al. Large-scale growth and field-effect transistors electrical engineering of atomic-layer SnS2[J]. Small, 2019, 15(46): 1904116.
[34] Ma Shouxiao, Jin Ying, Si Yang. Adsorption behavior of Pd-doped SnS2 monolayer upon H2 and C2H2 for dissolved gas analysis in transformer oil[J]. Adsorption, 2019, 25(8): 1587-1594.
[35] Guo Shiying, Hu Xuemin, Huang Yong, et al. A highly sensitive and selective SnS2 monolayer sensor in detecting SF6 decomposition gas[J]. Applied Surface Science, 2021, 541: 148494.
[36] Lin Long, Shi Zhengguang, Huang Jingtao, et al. Molecular adsorption properties of CH4 with noble metals doped onto oxygen vacancy defect of anatase TiO2 (1 0 1) surface: first-principles calculations[J]. Applied Surface Science, 2020, 514: 145900.
[37] Wanno B, Tabtimsai C. A DFT investigation of CO adsorption on VIIIB transition metal-doped graphene sheets[J]. Superlattices and Microstructures, 2014, 67: 110-117.
[38] Tabtimsai C, Rakrai W, Wanno B. Hydrogen adsorption on graphene sheets doped with group 8B transition metal: a DFT investigation[J]. Vacuum, 2017, 139: 101-108.
[39] Delley B. From molecules to solids with the DMol3 approach[J]. The Journal of Chemical Physics, 2000, 113(18): 7756-7764.
[40] Perdew J P, Burke K, Ernzerhof M. Generalized gradient approximation made simple[J]. Physical Review Letters, 1996, 77(18): 3865-3868.
Adsorption Properties of Os Doped SnS2 Monolayer to SOF2 and SO2F2
Abstract Based on density functional theory(DFT) of first-principles calculation in this paper, the gas-sensitive response of some decomposition gases such as SOF2 and SO2F2 on intrinsic SnS2 and Osn(n=1~2) modified SnS2 surface was explored. The gas sensing response mechanism of the three materials to SOF2 and SO2F2 was compared and analyzed from adsorption energy, band structure, DOS and HOMO-LUMO. It is found that intrinsic SnS2 has poor response to SOF2 and SO2F2. However, after doping,the Os modified position as active site on the surface of substrate materials effectively improves the gas sensitive response characteristics of the two gases on SnS2 surface: Osn-SnS2(n=1~2) have excellent performance on the adsorption of SOF2 and for SO2F2, Os2-SnS2 has ideal sensing properties. This study provides a theoretical basis for the experimental development of high-performance gas sensing sensors for detecting SF6 decomposing gases.
Keywords:Density functional theory(DFT), decomposition gases of SF6, Osn-SnS2, adsorption characteristics
DOI:10.19595/j.cnki.1000-6753.tces.210672
中图分类号:TM855
国家自然科学基金(51907165)、中央高校基本科研业务费专项资金(XDJK2020B024)资助项目。
收稿日期 2021-05-11
收稿日期 2021-07-29
桂银刚 男,1988年生,副教授,硕士生导师,研究方向为电力设备故障在线监测和新型纳米传感器。E-mail:yinganggui@swu.edu.cn (通信作者)
陈 盈 女,2000年生,硕士研究生,研究方向为电气设备在线监测、故障诊断及状态评价技术。E-mail:Chen_Ying_swu@163.com
(编辑 郭丽军)