磁场影响血糖和糖尿病并发症研究进展
冯传林1,2 郁 彪2,3 方彦雯4 方志财4 张 欣1,2,3
(1. 安徽大学物质科学与信息技术研究院 合肥 230601 2. 中国科学院合肥物质科学研究院强磁场科学中心 合肥 230031 3. 中国科学技术大学科学岛研究生分院 合肥 230026 4. 和也健康科技有限公司 湖州 313300)
摘要 多项研究表明,磁场会对机体血糖水平和糖尿病并发症产生一定影响。该文系统地比较并分析多种参数的磁场对多种生物系统的血糖、胰岛素水平以及糖尿病并发症的影响。研究结果显示,尽管磁场对血糖和胰岛素水平的影响会因为磁场参数和检测样品的多样化而导致其结果不同,但磁场对糖尿病伤口愈合以及骨关节病有着比较一致的正面效应,显示了良好的应用前景。虽然目前对磁场影响血糖的相关机制探索还不够系统深入,但已有证据表明,磁场可能通过影响细胞膜、膜蛋白以及Ca2+浓度等来影响胰岛素分泌。这不仅有助于人们深入了解磁场对血糖调节和糖尿病的影响,而且为未来进行更系统深入的研究,开发磁场在糖尿病及其并发症临床治疗中的潜在应用奠定基础。
关键词:磁场 血糖 胰岛素 糖尿病 糖尿病并发症
0 引言
磁场可以根据其强度和方向是否随时间变化而分为稳态磁场(Static Magnetic Fields, SMF)和动态磁场(Dynamic Magnetic Fields, DMF)。而根据其强度可进一步分为弱磁场(小于1mT)、中等磁场(1mT~1T)、强磁场(1~20T)和超强磁场(20T及以上)[1-3]。目前已有大量证据表明,磁场可以对多种生物体产生影响。例如,特定参数的脉冲磁场已被成功应用于经颅磁刺激,被全世界多个国家批准应用于临床,在治疗抑郁和癫痫等多方面发挥了独特的作用[4-7]。
糖尿病是一种以胰岛素分泌缺陷、作用缺陷或两者兼有而导致的以高血糖为特征的代谢性疾 病[8]。近年来,由于人口增长、老龄化、城市化、肥胖和缺乏体育锻炼等因素,糖尿病患者数量逐渐增加,严重危及人类健康并增加了社会医疗负担。而目前已有一些初步研究结果显示,一定条件下的磁场处理可能会对血糖调节产生影响,但由于实验条件不统一和数据不充分等,导致目前并无统一定论。本文旨在对相关研究进行总结和梳理,分析磁场对血糖和胰岛素等的影响,分析其机制,从而为进一步系统深入的研究奠定基础。
1 糖尿病简介
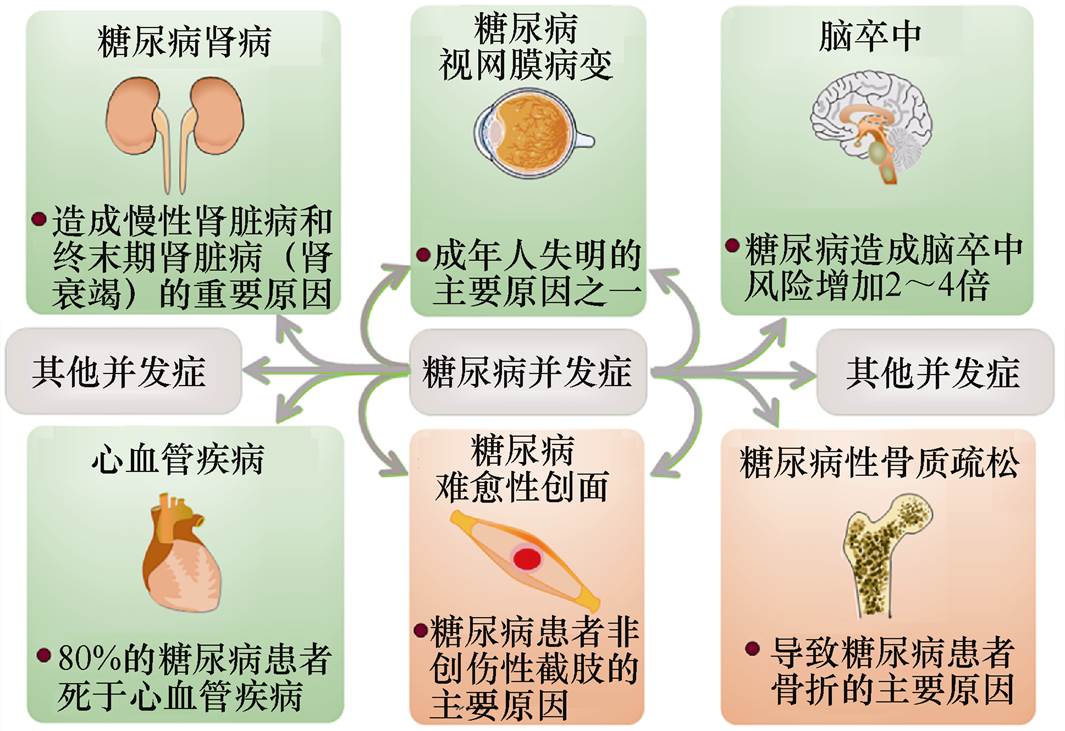
糖尿病是一种降低个体调节血液葡萄糖水平能力的代谢性疾病,其引起的慢性高血糖症可以对多种器官造成长期损害、功能障碍甚至衰竭,尤其是对视网膜、肾脏、神经、心脏和血管等,糖尿病相关并发症如图1所示。引起糖尿病的发病原因有很多,但主要原因包括胰岛细胞被自身免疫破坏所造成的胰岛素分泌缺乏以及胰岛素抵抗和胰岛素分泌反应不足等[8]。因此,根据糖尿病发病机制的不同,可以将糖尿病分为四种类型:①Ⅰ型糖尿病(Diabetes Mellitus Type 1, T1DM),又称胰岛素依赖型糖尿病或青少年型糖尿病,通常在儿童和青少年时期 (<35岁 发病;②Ⅱ型糖尿病(Diabetes Mellitus Type 2, T2DM),特点是胰岛素合成和分泌不足,约占全世界所有糖尿病的90%,其发病率可随着年龄的增长而增加;③妊娠糖尿病(Gestational Diabetes Mellitus, GDM),指的是妊娠期间发生并在妊娠期结束时消退的糖尿病;④青年成熟期发病型糖尿病(Maturity Onset Diabetes of the Young, MODY),是一种单基因型糖尿病,只占糖尿病患者总数很小的一部分,一般在20岁左右被诊断,与基因突变有 关[9]。此外,一些疾病和化学药物,如胰腺炎、甲状腺机能亢进、蛋白酶抑制剂和噻嗪类利尿剂也会造成一些继发性糖尿病的产生[9]。
发病;②Ⅱ型糖尿病(Diabetes Mellitus Type 2, T2DM),特点是胰岛素合成和分泌不足,约占全世界所有糖尿病的90%,其发病率可随着年龄的增长而增加;③妊娠糖尿病(Gestational Diabetes Mellitus, GDM),指的是妊娠期间发生并在妊娠期结束时消退的糖尿病;④青年成熟期发病型糖尿病(Maturity Onset Diabetes of the Young, MODY),是一种单基因型糖尿病,只占糖尿病患者总数很小的一部分,一般在20岁左右被诊断,与基因突变有 关[9]。此外,一些疾病和化学药物,如胰腺炎、甲状腺机能亢进、蛋白酶抑制剂和噻嗪类利尿剂也会造成一些继发性糖尿病的产生[9]。
2 磁场对血糖的影响
2012年,Y. Touitou等[10]在正常的地磁场条件下,对15名长期暴露在50Hz、大于3×10-7T的工频磁场下的成年男性与对照组人群进行对比,发现某些血液参数发生了具有统计学意义的变化,其中包括了血糖水平。除此之外,大多数磁场对血糖影响的研究都是在动物水平上,磁场对动物血糖的影响见表1。在13项相关研究中,有7项研究显示了磁场会升高机体的血糖水平(其中6项来自于Y. Tunisia的关于128mT的稳态磁场),3项研究显示降低血糖水平,3项显示无影响。
表1 磁场对动物血糖的影响
Tab.1 Effect of magnetic field on blood glucose in animals

研究对象磁场生理指标变化血糖总体效应文献 类型参数处理时间 雄性Wistar大鼠稳态磁场1mT,10mT1h/天,10天生长激素、促甲状腺素、甲状腺素、皮质醇、胰岛素水平降低升高[11] 雌性Wistar大鼠稳态磁场128mT1h/天,10天血小板、血红蛋白升高;天冬氨酸转氨酶和乳酸脱氢酶活性增加升高[12] 1h/天,13天红细胞压积、血红蛋白浓度升高、天冬氨酸转氨酶和乳酸脱氢酶活性增加;血浆胰岛素水平降低[13] 雄性Wistar大鼠稳态磁场128mT1h/天,15天血糖和乳酸升高,血浆胰岛素水平降低,甘油、胆固醇和磷脂增加;股四头肌和肝组织中的糖原含量显著降低升高[14] 体重、肝重、乳酸、胆固醇、磷脂和甘油三酯水平降低;低胰岛素血症[15] 稳态磁场128mT1h/天,5天,15天血浆中甘油、胆固醇、磷脂和乳酸水平显著升高;肝糖原含量降低;低胰岛素血症升高[16] 1h/天,5天血浆胰岛素水平下降[17] 雄性Wistar大鼠交变磁场 1.8~3.8mT,10Hz; 1.3~2.7mT,40Hz30min/天,1天、3天、6天、9天、14天胰岛素浓度升高;胰岛b 细胞超微结构发生可逆性改变降低[18] 5mT、8mT,50Hz165min/天,3周血浆总胆固醇和甘油三酯降低[19] 雄性 BALB/c小鼠稳态磁场-2.9×10-6~+2.9×10-6T24h/天,30天体重、血糖、血清总蛋白含量和碱性磷酸酶活性降低;乳酸脱氢酶、谷胱甘肽巯基转移酶活性增加降低[20] 交变磁场1.4mT,50Hz24h/天,30天体重、血糖、血清总蛋白含量和碱性磷酸酶活性降低;乳酸脱氢酶、谷胱甘肽巯基转移酶、肝脏g-谷氨酰转肽酶、肝脏谷胱甘肽巯基转移酶活性及硫代巴比妥酸反应物浓度显著增加降低[20] Wistar大鼠旋转磁场1 400r/min,35mT30min,15天血糖浓度与对照组比较没有显著差异无影响[21] 雌性白化BALB/c小鼠稳态磁场0.05T10h/天,25天体重和血糖浓度与对照组比较均无显著差异无影响[22] 雄性 db/db小鼠交变磁场50Hz,2mT2h/天,19天[23]
注:本文排除了少数几项具有明显缺陷的文献。
3 磁场对胰岛素的影响
不同参数的磁场对胰岛素水平的变化呈现出不同的作用效果见表2,在15项研究中,8项显示胰岛素释放减少,6项显示增高。但有趣的是,A. Hayek等[24]在研究稳态磁场对Sprague-Dawley大鼠离体胰岛功能的影响时发现,在0.1~1mT的磁感应强度下,比较低浓度(5.4mmol/L)葡萄糖条件下,磁场能够升高胰岛素水平,并且磁感应强度越高,效果越明显;但是在较高浓度(16.7mmol/L)葡萄糖条件下,效果并不明显。这说明磁场对胰岛素的影响与机体初始葡萄糖水平以及所用磁感应强度都直接相关。
4 磁场对糖尿病并发症的影响
4.1 磁场对糖尿病神经病变的生物学效应
糖尿病神经病变(Diabetic Neuropathy, DN)是糖尿病患者排除其他原因后出现外周神经功能损害的一种病症[32],是糖尿病所有长期并发症中最常见的一种。大约50%的糖尿病患者会出现疼痛性糖尿病神经病变,症状包括超敏、自发性疼痛、麻木和痛觉过敏[33-34]。高血糖会引起中枢或外周神经系统结构损伤或功能异常[35],是糖尿病神经病变最重要的致病因素。目前关于磁场对糖尿病神经病变的影响见表3。例如,对于糖尿病患者的人体实验共有四项相关研究,发现其中两项可以对其神经病变产生缓解效果[36-37],而另外两项则无效果[38-39]。相比之下,三项中等强度低频脉冲磁场的动物研究都显示出了正面效果[40-42]。
表2 磁场对胰岛素的影响研究
Tab.2 Effect of magnetic field on insulin

研究对象磁场对胰岛素的影响文献 类型参数处理时间具体影响总体效应 雄性Wistar大鼠稳态磁场1mT,10mT1h/天,10天血糖浓度升高;血浆胰岛素水平降低,出现低胰岛素血症胰岛素释放量减少[11] 128mT1h/天,13天[13] 1h/天,15天[14] [15] 1h/天,5/15天[16] 1h/天,5天[17] 兔离体胰岛细胞脉冲磁场强度、频率不明18h胰岛b 细胞内游离Ca2+含量降低,Ca2+外排减少[25] HITT15细胞交变磁场5mT,60Hz2h200mg/dL葡萄糖刺激下,抑制细胞膜去极化,ATP/ADP减少,胞浆游离Ca2+浓度降低[26] 雄性Wistar大鼠交变磁场0.24A/m,1.9GHz7h/天,30天胰腺表面外分泌和内分泌细胞的数量增加;胰腺细胞绝对表面积增加胰岛素分泌增加[27] HITT15细胞5mT,60Hz2天、5天40和100mg/dL葡萄糖刺激下,细胞凋亡数量减少[28] 雄性Wistar大鼠离体胰岛细胞1.3~3.8mT,10/40Hz30min/天,1天、3天、 6天、9天、14天胰岛细胞高尔基体和粗面内质网扩张、线粒体肿胀、胰岛b 细胞空泡增多[18] INS-1细胞稳态磁场6T1h胰岛素mRNA表达增加[29] 400mT6h、12h、18h[30] 400mT12h、18h、24h、48h、 72h上调胰腺特异性转录因子和囊泡分泌蛋白的表达,增强胰岛素基因启动子活性,胰岛素基因表达增强,促进胰岛素分泌[31] Sprague-Dawley孕鼠离体胰岛细胞稳态磁场0.1~1mT48h高浓度葡萄糖,抑制释放胰岛素;低浓度葡萄糖,胰岛素释放量与磁场强度成正比胰岛素分泌情况与葡萄糖水平和磁场强度相关[24]
表3 磁场对糖尿病神经病变的影响研究
Tab.3 Effect of magnetic field on diabetic neuropathy

研究对象磁场磁场对糖尿病神经病变的影响文献 类型参数处理时间具体影响总体效应 糖尿病神经病变患者的足部稳态磁场45mT24h/天,4个月灼热感、麻木感、刺痛感和运动引起的足部疼痛均有所降低症状缓解[36] 糖尿病患者的上肢和下肢脉冲磁场 100Hz、10Hz, 8mT 10次/天, 10~15min/次改善周围神经传导功能,改善了1A传入纤维状态,改善脊髓功能多样性运动神经元的反射兴奋性症状缓解[37] 雄性Sprague- Dawley糖尿病大鼠15Hz,1.6mT8h/天,7周显著抑制有害热刺激和机械刺激的超敏反应,可部分阻止链脲佐菌素处理的糖尿病周围神经病变大鼠轴突变性的发展[40] 雄性Wistar大鼠脉冲磁场 1Hz、5Hz、30Hz、40Hz,1.5mT1h/天,5周提高疼痛潜伏期和阈值;显著降低脊髓内大部分促炎细胞因子的水平,并具有抗痛觉和抗超敏作用症状缓解[41] 1Hz、3Hz、5Hz、7Hz, 1.5mT1h/天,4周可阻止糖尿病大鼠热刺激缩足反射潜伏期和机械刺激阈值的增加;坐骨和尾神经的运动神经传导速度明显恢复;坐骨神经和脊髓组织中氧化应激程度有所降低[42] 糖尿病周围神经病变患者脉冲磁场 180~195Hz, 0.1mT1次/天,20min/次, 15天疼痛强度、周围神经传导速度、诱发电位振幅和潜伏期与对照组相比均无统计学差异无影响[38] 糖尿病患者的足部频率不明,180mT2h/天,3个月曝磁组和假曝磁组在神经病理性疼痛的强度上无显著性差异[39]
4.2 磁场对糖尿病小鼠伤口愈合的影响
糖尿病性皮肤溃疡所造成的伤口往往难以愈合,是糖尿病患者入院、截肢和死亡的主要原因,给患者带来了极大的痛苦[43]。而动物实验研究表明,多种参数的磁场都有可能作为一种无创的物理方法,来加快糖尿病小鼠的伤口愈合,见表4。例如,就稳态磁场而言,2013年,聂志勇等[48]使用直径为3cm、磁感应强度为300mT的钕铁硼永磁片粘附在糖尿病大鼠创面的纱布上,发现可以促进其伤口愈合速度,并且伤口组织的抗张强度亦显著提升。就脉冲磁场而言,2015年,M. C. Choi等[54]发现磁感应强度为5mT、频率为25Hz的脉冲电磁场(Pulsed Electromagnetic Fields, PEMF)可以加速伤口早期的愈合、增加胶原沉积以及增强伤口闭合处瘢痕组织的抗拉伸强度,从而对糖尿病创面早期愈合过程产生有益作用,但对伤口中后期愈合过程没有明显影响。就交变磁场而言,2020年,郭毅敏等[23]探究了输出波形为50Hz、峰值磁感应强度为2mT的正弦波交变电磁场对Ⅱ型糖尿病db/db小鼠创面愈合修复影响,发现低强度的正弦波交变电磁场可以显著加快小鼠创伤修复并提高创伤组织的抗张强度。
表4 磁场对糖尿病伤口愈合的影响研究
Tab.4 Effect of magnetic field on diabetic wound healing

研究对象磁场磁场对糖尿病伤口愈合的影响文献 类型参数处理时间具体影响总体效应 雄性Sprague- Dawley大鼠稳态磁场180mT24h/天,5~19天炎症细胞数量和坏死水平显著减少;愈合率显著提高,总愈合时间缩短;胶原沉积量和创面抗张强度有较大幅度提高促进愈合[44] 缩短创伤愈合时间,提升软组织创伤愈合率[45] 雄性Wistar大鼠稳态磁场180mT24h/天,3天、7天创缘红肿消退快速,肉芽组织快速增多,创面愈合率增高;毛细血管内皮细胞体积增大,促进血管增生,加速血液循环;创面局部炎症微环境出现明显改变促进愈合[46] 230mT24h/天,7~21天创面面积缩小率显著加快,创面愈合总时间缩短,伤口组织强度和应激程度显著提高[47] 雌性Sprage- Dawley大鼠稳态磁场300mT24h/天,6~20天伤口软组织抗张强度提升,愈合时间缩短,愈合率提升促进愈合[48] 雄性db/db小鼠稳态磁场0.6T24h/天,14天加速伤口愈合,促进再上皮化、血管重建和炎症的消退,上调抗炎基因的表达促进愈合[49] 4mT1h/天,5~19天缩短创伤总愈合时间,伤口抗张强度显著加强,炎性细胞显著减少,伤口组织中白细胞介素-1b、白细胞介素-6和肿瘤坏死因子-a 的表达水平显著降低[50] db/db和C57BL6小鼠脉冲磁场15Hz,20mT8h/天,直至愈合显著加快糖尿病鼠和正常鼠伤口闭合;血管密度增加;含有CD31的细胞和内皮细胞数量显著增加;碱性成纤维细胞生长因子-2表达上调促进愈合[51] 雄性Wistar大鼠脉冲磁场20Hz,8mT1h/天,10天缩短了愈合时间,提高伤口愈合率,伤口抗张强度增强促进愈合[52] 雄性Sprague- Dawley大鼠脉冲磁场25Hz,5mT1h/天,21天伤口面积缩小快,伤口愈合率高;肌成纤维细胞数量多;肌成纤维细胞明显多于对照组促进愈合[53] 25Hz,5mT1h/天,7~14天I型胶原纤维沉积稳步增加,沉积量均大于对照组;肌成纤维细胞数量明显增多[54] 25Hz,2/10mT1h/天,3~21天创面加速愈合,伤口厚度增加,拉伸强度提高[55] 雄性db/db小鼠交变磁场50Hz,2mT2h/天,5~19天创伤总愈合时间降低,抗张强度增加,血流速率增加;伤口组织中血管内皮生长因子,血管生成素-l和胰岛素样生长因子-1蛋白表达增加促进愈合[23]
4.3 磁场对糖尿病骨关节病的影响
由于糖尿病可显著损害骨的形成,削弱骨的机械强度,加速骨骼结构的退化[56-57],因此糖尿病患者较容易发生骨折[58]、骨折后难以愈合[59]和骨质疏松[60]等。并且由于糖尿病患者的组织再生能力较差,因而由骨折导致的死亡率明显高于非糖尿病人群[61]。因此开发一种针对糖尿病骨关节病更安全、更有效的防治方法具有重要的临床意义。目前已有多项研究表明,多种类型的磁场可以对糖尿病骨关节病(Diabetic Osteoarthropathy, DOAP)产生有益的影响,见表5。例如,就稳态磁场而言,2018年,Zhang Hao等[63]研究发现,Ⅰ型糖尿病大鼠全身暴露于4mT的稳态磁场中16周后可抑制小鼠骨小梁和皮质骨的结构退化和机械强度的降低,并且可以使小鼠血清骨钙素、骨小梁骨矿物质沉积率和成骨细胞数增加,同时成骨细胞骨钙素、骨形成蛋白-2(Bone Morphogenetic Protein 2, BMP2)和Runx2的基因表达也有所增强,提示适度的稳态磁场通过抑制Ⅰ型糖尿病小鼠骨损失来防止骨结构的退化和强度的降低。就脉冲磁场而言,2018年,Cai Jing等[66]报道了频率为15Hz、强度峰值为2mT的PEMF对Ⅰ型糖尿病兔骨的影响,研究也发现了PEMF刺激下糖尿病兔骨髓腔变窄、增加皮质骨厚度以及松质骨数量,缓解了松质骨和皮质骨结构及组织水平机械强度的退化,PEMF使骨形成标志物血清骨钙素(Osteocalcin, OCN)和血清Ⅰ型前胶原肽(Propeptide of type1 Procollagen, P1NP)浓度显著升高,促进了骨的整合和生长,此外,PEMF还上调了股骨OCN、BMP2和Runx2 mRNA的表达,激活T1DM骨骼中与成骨相关的Wnt/b-catenin信号,并显著减轻Ⅰ型糖尿病引起的骨形成的减少。
表5 磁场对糖尿病骨关节病的影响研究
Tab.5 Effect of magnetic field on diabetic osteoarthritis

研究对象磁场磁场对糖尿病骨关节病的影响文献 类型参数处理时间具体影响总体效应 糖尿病Charcot关节病患者复合磁场频率、强度不明1/2h,112h显著缩短恢复时间,减少骨关节损坏和残余的畸形数量促进[62] 雄性Sprague- Dawley大鼠稳态磁场4mT2h/天,16周血清骨钙素浓度显著高于未暴露的糖尿病大鼠;骨的最大负荷、硬度和韧性都有所提升;成骨相关基因表达增强促进[63] 脉冲磁场15Hz,2.4mT8h/天,8周提高骨机械强度,表现为最大载荷、刚度和能量吸收水平增加,阻止小梁骨质量和微结构的恶化[64] 雄性db/db小鼠脉冲磁场15Hz,2mT2h/天,12周骨形成关键标志物血清骨钙素浓度显著升高;骨小梁和皮质微结构明显改善;骨硬度和承载力增加;骨形成相关基因表达上调促进[65] 雄性New Zealand兔子脉冲磁场15Hz,2mT2h/天,8周胫骨最大承载力、弹性、硬度提升;骨形成标志物血清骨钙素和血清Ⅰ型前胶原肽浓度升高,促进糖尿病兔骨骼中Wnt3a、Lrp6和b-catenin基因表达促进[66]
5 磁场影响糖尿病的可能机制
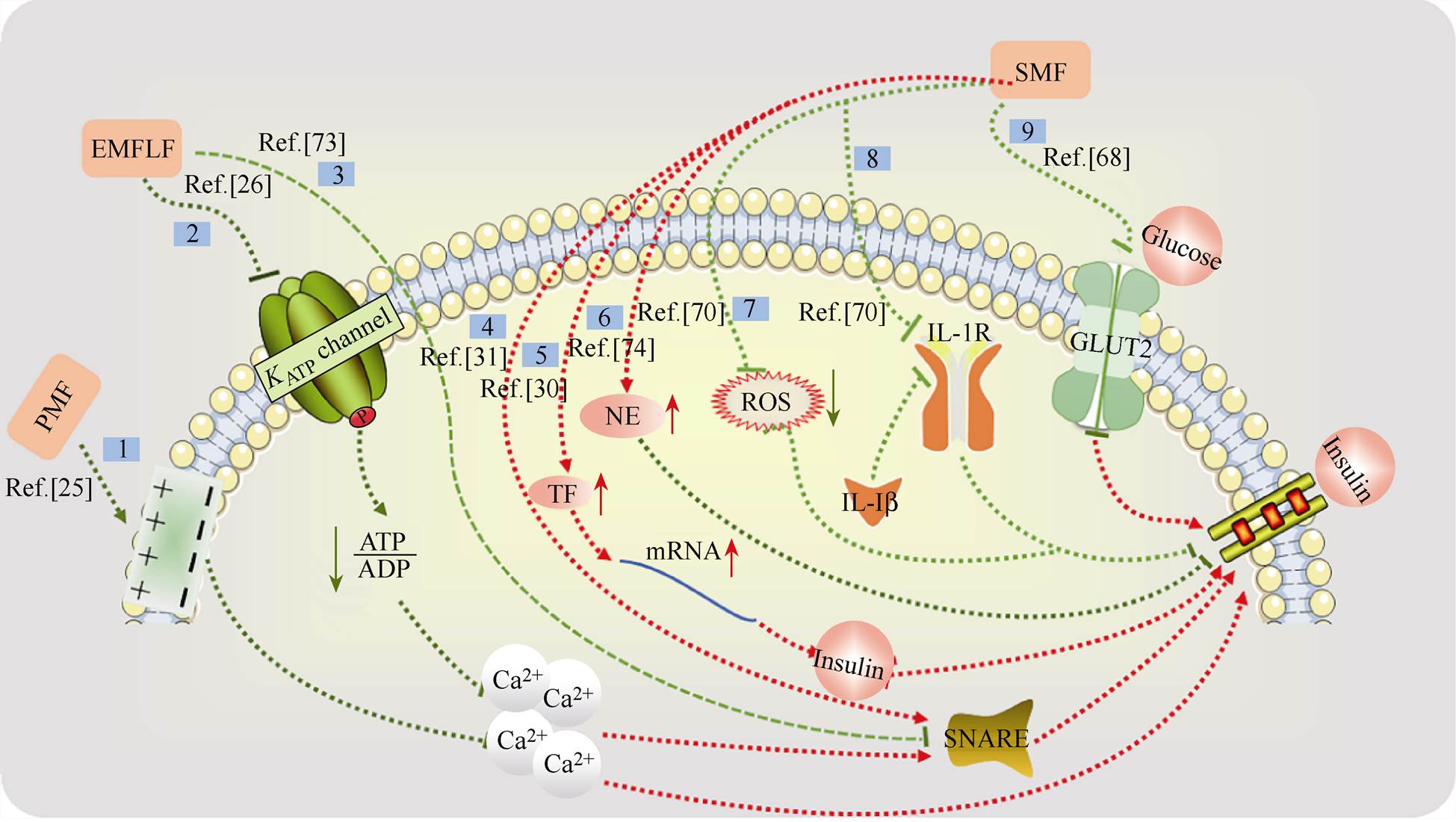
目前对于磁场对糖尿病的影响的机制已有一些初步探索,如图2所示。但是由于不同研究所用磁场参数和研究对象大多不同,所以对磁场影响糖尿病的机制目前尚无定论。例如,胰岛b 细胞是调节血糖平衡的重要细胞,它可以释放胰岛素从而促进血液循环中葡萄糖合成糖原使血糖降低,促进脂肪及蛋白质的合成,因此胰岛b 细胞释放胰岛素是血糖调节的关键步骤。目前有多项研究表明,磁场可以影响胰岛b 细胞的细胞膜及离子通道[25-26, 67]。但除胰岛细胞外,人们还发现了其他一些关于磁场调节血糖或胰岛素的机制。例如,通过胰高血糖素、皮质醇、甲状腺激素和生长激素[11]、儿茶酚胺、葡萄糖转运蛋白[68-69]以及白细胞介素-1b(Interleukin-1b, IL-1b)受体[70],如图2所示。图中,途径1,脉冲磁场(Pulsed Magnetic Field, PMF)产生的电场梯度使细胞膜上钙离子结合位点发生改变,胰岛b 细胞内游离Ca2+的含量降低,胰岛素释放减少[25]。 途径2,极低频磁场(Extremely Low Frequency Magnetic Field, ELFMF)使腺嘌呤核苷三磷酸(Adenosine Triphosphate, ATP)与腺嘌呤核苷二磷酸(Adenosine Diphosphate, ADP)之间的比值降低,ATP依赖性钾离子通道关闭,阻止膜去极化,引起电压依赖性钙离子通道的关闭,胞浆游离Ca2+浓度降低,胰岛素无法分泌[26]。途径3,可溶性N-乙基马来酰亚胺敏感因子附着蛋白受体(Soluble N- ethylmaleimide-sensitive fusion protein Attachment protein Receptor, SNARE)蛋白复合物可以帮助胰岛素转运出细胞,而ELFMF会降低SNARE蛋白复合物组成部分突触小体相关蛋白25(Synaptosomal- Associated Protein 25, SNAP 25)和突触结合蛋白1(Synaptotagmin 1, Syt 1)的mRNA表达水平,使胰岛素无法释放[71]。途径4,SMF的刺激通过调节一些SNARE囊泡蛋白的表达来调节胰岛素的分泌[31]。途径5,SMF下诱导多种转录因子的表达,转录因子与胰岛素基因的启动子区域结合,并促进胰岛素基因的表达[30]。途径6,稳态磁场促使去甲肾上腺素水平升高,而去甲肾上腺素会干扰胰岛素的释放[72]。途径7,氧化应激会破坏胰岛素生成,SMF处理后可以降低氧化应激,促进胰岛素生成[70]。途径8,SMF处理后类似于白细胞介素-1b 受体(Interleukin-1 Receptor, IL-1R)拮抗剂的效果,降低IL-1b 影响,减少先天免疫细胞的募集,协助胰岛素释放,从而降低血糖[70]。途径9,稳态磁场可能破坏胰岛b 细胞中的葡萄糖转运蛋白2(Glucose Transporters 2, GLUT2),胰岛b 细胞无法感知外界葡萄糖浓度,胰岛素无法顺利释放[68]。
在目前的为数不多的机制研究中,关于稳态磁场的研究都集中在生物学机制的探索上。而在动态磁场方面,发现动态磁场引起的膜电位和电压依赖性离子通道可能通过降低钙离子来影响胰岛素分泌[25-26](见图2)。此外,有研究发现,波形为矩形的脉冲磁场通常会导致生物膜的结构特性和渗透性发生更加显著的变化[73-74],A. Laitl-Kobierska等[18]认为这种效应很可能是造成磁场下胰岛素分泌过程更加剧烈的原因。未来本领域亟需在生物物理和生物电磁的角度对磁场影响糖尿病的机制进行系统深入的研究,从而找出导致其生物学效应的根源。
6 磁场影响糖尿病效果不统一原因分析
目前磁场与血糖或胰岛素的实验结果多种多样(见表1和表2)可能与不同研究中所采用的曝磁装置(见图3)产生的磁场参数(强度、梯度、方向、波形)、处理方式(曝磁时间、时间间隔、总时长)、所研究对象以及联合治疗的方法等多因素有关。例如,磁场梯度与磁感应强度的乘积对生物效应起着关键的作用[71],可以影响细胞成分和培养基中溶解氧等的分布等[75-76]。相关研究所用的不同磁场装置如图3所示。图3a为Lake Shore电磁铁装置产生稳态磁场,磁感应强度为128mT[13];图3b为交变磁场系统,磁感应强度为1.4mT,频率为50Hz[20];图3c为由一块钕铁硼磁铁提供的非均匀稳态磁场,表面磁感应强度为0.6T[49];图3d为脉冲磁场装置,磁感应强度为2mT,频率为15Hz[65];图3e为由两块组合磁板(内含多个1cm直径和1cm高的圆柱形钕铁硼小磁铁)提供的非均匀稳态磁场,磁感应强度在2.8~476.7mT范围内[70]。
2009年,T. Sakurai等[29]研究发现,在磁感应强度为6T时可以显著提高胰岛素分泌水平,并通过检测和计算磁场梯度与磁感应强度的乘积发现,在其研究系统中,6T时的磁场梯度与磁感应强度乘积最高。又如,动态磁场参数中的波形也会影响胰岛素释放,以及生物体初始血糖水平和磁感应强 度[24],磁场空间分布和磁场处理时间等都可能会直接影响磁场效果。此外,最近C. S. Carter等发现,3mT稳态磁场和7kV/m稳态电场联合作用于多种Ⅱ型糖尿病鼠可以显著改善其血糖水平和胰岛素抵抗;但当仅用3mT磁场处理这些糖尿病鼠模型时会恶化其血糖水平和葡萄糖耐受[77]。而本课题组发现,稳态磁感应强度、方向和分布可以直接导致对糖尿病小鼠血糖调节的不同作用(未发表数据)。因此对于推进磁场与糖尿病相关标准化研究,建议研究者按照所要研究糖尿病类型构建相应的糖尿病动物模型,再经一定参数的磁场处理(准确标明实验动物所处磁场环境,包括磁感应强度、方向、分布、频率、波形等多种磁场参数,以及磁场处理方式和时间),然后根据所要研究的糖尿病并发症的特点来决定具体检测的实验参数。建议检测的基本参数包括糖尿病小鼠体重、饮食和血糖变化曲线。其他可根据并发症类型和实验条件,如对胰岛素水平、骨密度、血管生成相关指标(例如肾小球毛细血管和血管生成标志物)等进行检测。其中,磁场参数是最为关键的因素,此外,所用实验动物的性别、年龄、品种等多种关键因素也需明确记录。
7 结论
总体而言,虽然由于磁场本身参数以及生物样品的不同导致了目前有关磁场调节血糖和糖尿病症状的作用大多无确凿定论。但从目前的数据看来,磁场在改善糖尿病的特定并发症,尤其在减少伤口愈合时间和改善糖尿病骨病等方面有着非常正面的效果。然而与此同时,也存在一些磁场条件会升高血糖和减少胰岛素释放。因此,需要从多方面研究不同磁场条件(包括磁场类型、强度、方向和处理时间等)对各种生物样品(如细胞类型、形态和动物种类,以及不同血糖浓度起始状态等)造成不同影响的原因,深入探索其分子机制,优化磁场条件,从而推动未来磁场在调节血糖和缓解糖尿病并发症等方面的临床应用。
参考文献
[1] 张欣, Yarema K, 许安. 稳态磁场的生物学效应[M]. 北京: 科学出版社, 2018.
[2] Zhang Xin, Yarema K, Xu An. Biological effects of static magnetic fields[M]. Singapore: Springer, 2017.
[3] Zhang Xin, Wang J F. Interdisciplinary research of magnetic fields and life sciences[M]. Beijing: Science Press, 2018.
[4] Lefaucheur J P, Aleman A, Baeken C, et al. Evidence- based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014-2018)[J]. Clinical Neurophysiology, 2020, 131(2): 474-528.
[5] 李达, 许毅, 安建雄. 重复经颅磁刺激治疗专家共识[J]. 转化医学杂志, 2018, 7(1): 4-9.
Li Da, Xu Yi, An Jianxiong. Chinese experts con- sensus on repetitive transcranial tagnetic stimulation[J]. Translational Medicine Journal, 2018, 7(1): 4-9.
[6] Zhou Xiaoqing, Liu Shikun, Wang Yuexiang, et al. High-resolution transcranial electrical simulation for living mice based on magneto-acoustic effect[J]. Frontiers in Neuroscience, 2019, 13(1342): 1-12.
[7] Wang Huiqin, ZhouXiaoqing, Cui Dong, et al. Comparative study of transcranial magneto-acoustic stimulation and transcranial ultrasound stimulation of motor cortex[J]. Frontiers in Behavioral Neuroence, 2019, 13(241): 1-10.
[8] Assoc A D. Diagnosis and classification of diabetes mellitus[J]. Diabetes Care, 2010, 33(S1): 62-69.
[9] Kaul K, Tarr J M, Ahmad S I, et al. Introduction to diabetes mellitus[J]. Advances in Experimental Medicine and Biology, 2012, 771: 1-11.
[10] Touitou Y, Djeridane Y, Lambrozo J, et al. Long-term (up to 20 years) effects of 50Hz magnetic field exposure on blood chemistry parameters in healthy men[J]. Clinical Biochemistry, 2012, 45(6): 425-428.
[11] Gorczynska E, Wegrzynowicz R. Glucose-homeostasis in rats exposed to magnetic-fields[J]. Investigative Radiology, 1991, 26(12): 1095-1100.
[12] Sihem C, Hafedh A, Mohsen S, et al. Effects of sub-acute exposure to magnetic field on blood hematological and biochemical parameters in female rats[J]. Turkish Journal of Hematology, 2006, 23(4): 182-187.
[13] Chater S, Abdelmelek H, Pequignot J M, et al. Effects of sub-acute exposure to static magnetic field on hematologic and biochemical parameters in pregnant rats[J]. Electromagnetic Biology & Medicine, 2006, 25(3): 135-144.
[14] Elferchichi M, Mercier J, Coisy-Quivy M, et al. Effects of exposure to a 128mT static magnetic field on glucose and lipid metabolism in serum and skeletal muscle of rats[J]. Archives of Medical Research, 2010, 41(5): 309-314.
[15] Elferchichi M, Mercier J, Bourret A, et al. Is static magnetic field exposure a new model of metabolic alteration? comparison with Zucker rats[J]. Inter- national Journal of Radiation Biology, 2011, 87(5): 483-490.
[16] Lahbib A, Elferchichi M, Ghodbane S, et al. Time- dependent effects of exposure to static magnetic field on glucose and lipid metabolism in rat[J]. General Physiology and Biophysics, 2010, 29(4): 390-395.
[17] Lahbib A, Ghodbane S, Maaroufi K, et al. Vitamin D supplementation ameliorates hypoinsulinemia and hyperglycemia in static magnetic field-exposed rat[J]. Archives of Environmental & Occupational Health, 2015, 70(3): 142-146.
[18] Laitl-Kobierska A, Cieslar G, Sieron A, et al. Influence of alternating extremely low frequency ELF magnetic field on structure and function of pancreas in rats[J]. Bioelectromagnetics, 2002, 23(1): 49-58.
[19] Ocal I, Kalkan T, Gunay I. Effects of alternating magnetic field on the metabolism of the healthy and diabetic organisms[J]. Brazilian Archives of Biology and Technology, 2008, 51(3): 523-530.
[20] Hashish A H, El-Missiry M A, Abdelkader H I, et al. Assessment of biological changes of continuous whole body exposure to static magnetic field and extremely low frequency electromagnetic fields in mice[J]. Ecotoxicology & Environmental Safety, 2008, 71(3): 895-902.
[21] 韩丽莎, 王芳. 旋磁场对糖代谢影响的研究[J]. 中华理疗杂志, 1997, 20(4): 231-232.
Han Lisha, Wang Fang. Effect of rotating magnetic field on glucose metabolism[J]. Chinese Journal of Physical Therapy, 1997, 20(4): 231-232.
[22] Abbasi M, Nakhjavani M, Hamidi S, et al. Constant magnetic field of 50mT does not affect weight gain and blood glucose level in BALB/c mice[J]. Medical Science Monitor, 2007, 13(7): 151-154.
[23] 郭毅敏, 汪天赐, 蔡婧, 等. 正弦波交变电磁场对2型糖尿病皮肤软组织创伤愈合修复的研究[J]. 成都医学院学报, 2020, 15(1): 13-18.
Guo Yimin, Wang Tianci, Cai Jing, et al. Study on the effect of sinusoidal alternating electromagnetic fields on the wound healing and repair of soft tissues in patients with type 2 diabetes mellitus[J]. Journal of Chengdu Medical College, 2020, 15(1): 13-18.
[24] Hayek A, Guardian C, Guardian J, et al. Homo- geneous magnetic fields influence pancreatic islet function in vitro[J]. Biochemical & Biophysical Research Communications, 1984, 122(1): 191-196.
[25] Jolley W B, Hinshaw D B, Knierim K, et al. Magnetic field effects on calcium efflux and insulin secretion in isolated rabbit islets of Langerhans[J]. Bioelectro- magnetics, 1983, 4(1): 103-106.
[26] Sakurai T, Koyama S, Komatsubara Y, et al. Decrease in glucose-stimulated insulin secretion following expo- sure to magnetic fields[J]. Biochemical & Biophysical Research Communications, 2005, 332(1): 28-32.
[27] Paras S D, Gajanin R B, Manojlovi M L, et al. Impact of high-frequency electromagnetic fields on secretion and structure of pancreas in rats [C]//European Medical and Biological Engineering Confernce Nordic-Baltic Conference on Biomedical Engineering and Medical Physics, Tampere, Finland, 2017: 711-714.
[28] Sakurai T, Yoshimoto M, Koyama S, et al. Exposure to extremely low frequency magnetic fields affects insulin-secreting cells[J]. Bioelectromagnetics, 2008, 29(2): 118-124.
[29] Sakurai T, Terashima S, Miyakoshi J. Effects of strong static magnetic fields used in magnetic resonance imaging on insulin-secreting cells[J]. Bioelectromagnetics, 2009, 30(1): 1-8.
[30] Mao Libin, Guo Zhixia, Wang Huiqin, et al. Exposure to static magnetic fields affects insulin secretion in ins cells[J]. Lecture Notes in Electrical Engineering, 2015, 333: 643-648.
[31] Mao Libin, Wang Huiqin, Ma Fenghui, et al. Exposure to static magnetic fields increases insulin secretion in rat INS-1 cells by activating the transcription of the insulin gene and up-regulating the expression of vesicle-secreted proteins[J]. International Journal of Radiation Biology, 2017, 93(8): 831-840.
[32] Boulton A J, Gries F A, Jervell J A. Guidelines for the diagnosis and outpatient management of diabetic peripheral neuropathy[J]. Diabetic Medicine, 1998, 15(6): 508-514.
[33] Said G. Diabetic neuropathy-a review[J]. Nature Clinical Practice Neurology, 2007, 3(6): 331-340.
[34] Yagihashi S, Yamagishi S, Wada R. Pathology and pathogenetic mechanisms of diabetic neuropathy: correlation with clinical signs and symptoms[J]. Diabetes Research & Clinical Practice, 2007, 77(S1): 184-189.
[35] Obrosova I G. Diabetes and the peripheral nerve[J]. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease, 2009, 1792(10): 931-940.
[36] Weintraub M I, Wolfe G I, Barohn R A, et al. Static magnetic field therapy for symptomatic diabetic neuropathy: a randomized, double-blind, placebo- controlled trial[J]. Archives of Physical Medicine and Rehabilitation, 2003, 84(5): 736-746.
[37] Musaev A V, Guseinova S G, Imamverdieva S S. The use of pulsed electromagnetic fields with complex modulation in the treatment of patients with diabetic polyneuropathy[J]. Neuroence & Behavioral Physiology, 2003, 33(8): 745-752.
[38] Wrobel M P, Szymborska-Kajanek A, Wystrychowski G, et al. Impact of low frequency pulsed magnetic fields on pain intensity, quality of life and sleep disturbances in patients with painful diabetic poly- neuropathy[J]. Diabetes & Metabolism, 2008, 34(4 Pt 1): 349-354.
[39] Weintraub M I, Herrmann D N, Smith A G, et al. Pulsed electromagnetic fields to reduce diabetic neuropathic pain and stimulate neuronal repair: a randomized controlled trial[J]. Archives of Physical Medicine and Rehabilitation, 2009, 90(7): 1102-1109.
[40] Lei Tao, Jing Da, Xie Kangning, et al. Therapeutic effects of 15Hz pulsed electromagnetic field on diabetic peripheral neuropathy in streptozotocin- treated rats[J]. PLoS One, 2013, 8(4): 1-9.
[41] Mert T, Gisi G, Celik A, et al. Frequency-dependent effects of sequenced pulsed magnetic field on experimental diabetic neuropathy[J]. International Journal of Radiation Biology, 2015, 91(10): 833-842.
[42] Mert T, Sahin E, Yaman S, et al. Pulsed magnetic field treatment ameliorates the progression of peripheral neuropathy by modulating the neuronal oxidative stress, apoptosis and angiogenesis in a rat model of experimental diabetes[J]. Archives of Physiology and Biochemistry, 2020, https://doi.org/10.1080/13813455. 2020.1788098.
[43] Motley T A, Gilligan A M , Lange D L, et al. Cost- effectiveness of clostridial collagenase ointment on wound closure in patients with diabetic foot ulcers: economic analysis of results from a multicenter, randomized, open-label trial[J]. Journal of Foot and Ankle Research, 2016, 9(1): 1-7.
[44] Jing Da, Shen Guanghao, Cai Jing, et al. Effects of 180mT static magnetic fields on diabetic wound healing in rats[J]. Bioelectromagnetics, 2010, 31(8): 640-648.
[45] 孙涛, 罗二平, 申广浩, 等. 静磁场对糖尿病大鼠表皮创伤的抑制作用[J]. 医疗卫生装备, 2014, 35(4): 4-6.
Sun Tao, Luo Erping, Shen Guanghao, et al. Inhibitive effects of static magnetic field on wound healing in diabetes rat[J]. Chinese Medical Equipment Journal, 2014, 35(4): 4-6.
[46] 雷霆, 孟昕珂, 魏海梁, 等. 磁疗对糖尿病足溃疡大鼠模型创面愈合的影响[J]. 世界中西医结合杂志, 2020, 15(4): 638-641.
Lei Ting, Meng Xinke, Wei Hailiang, et al. Effect of magnet therapy on wound healing in rat models with diabetic foot ulcer: an experimental study[J]. Word Journal of Integrated Traditional and Western Medicine, 2020, 15(4): 638-641.
[47] Zhao Jing, Li Yongguo, Deng Kaiqin, et al. Therapeutic effects of static magnetic field on wound healing in diabetic rats[J]. Journal of Diabetes Research, 2017: 6305370.
[48] 聂志勇, 邢柏春, 李耀明, 等. 300mT恒磁场对于糖尿病大鼠创伤愈合的影响[J]. 现代生物医学进展, 2013, 13(20): 3801-3803.
Lie Zhiyong, Xing Bochun, Li Yaoming, et al. Effects of 300mT static magnetic fields on diabetic wound healing in rats[J]. Progress in Modern Biomedicine, 2013, 13(20): 3801-3803.
[49] Shang Wenlong, Chen Guilin, LiYinxiu, et al. Static magnetic field accelerates diabetic wound healing by facilitating resolution of inflammation[J]. Journal of Diabetes Research, 2019(2): 1-11.
[50] 刘丹, 汪天赐, 景达, 等. 中等强度的全身性稳恒磁场暴露对于2型糖尿病软组织创伤愈合修复的实验研究[J]. 海南医学院学报, 2019, 25(19): 1441- 1446.
Liu Dan, Wang Tianci, Jing Da, et al. Repairing effects of moderate-intensity static magnetic fields on wound healing of type 2 diabetic soft tissue[J]. Journal of Hainan Medical University, 2019, 25(19): 1441-1446.
[51] Callaghan M J, Chang E I, Seiser N, et al. Pulsed electromagnetic fields accelerate normal and diabetic wound healing by increasing endogenous FGF-2 release[J]. Plastic & Reconstructive Surgery, 2008, 121(1): 130-141.
[52] Goudarzi I, Hajizadeh S, Salmani M E, et al. Pulsed electromagnetic fields accelerate wound healing in the skin of diabetic rats[J]. Bioelectromagnetics, 2010, 31(4): 318-323.
[53] Cheing G L, Li Xiaohui, Huang Lin, et al. Pulsed electro-magnetic fields (PEMF) promote early wound healing and myofibroblast proliferation in diabetic rats[J]. Bioelectromagnetics, 2014, 35(3): 161-169.
[54] Choi M C, Cheung K K, Li Xiaohui, et al. Pulsed electromagnetic field (PEMF) promotes collagen fibre deposition associated with increased myofibroblast population in the early healing phase of diabetic wound[J]. Archives of Dermatological Research, 2016, 308(1): 21-29.
[55] Choi H M C, Cheing A K K, Ng G Y F, et al. Effects of pulsed electromagnetic field (PEMF) on the tensile biomechanical properties of diabetic wounds at different phases of healing[J]. PLoS One, 2018, 13(11): 1-12.
[56] Krakauer J C, Mckenna M J, Buderer N F, et al. Bone loss and bone turnover in diabetes[J]. Diabetes, 1995, 44(7): 775-782.
[57] Botolin S, Mccabe L R. Bone loss and increased bone adiposity in spontaneous and pharmacologically induced diabetic mice[J]. Endocrinology, 2007, 148(1): 198-205.
[58] Heath H, Melton L J, Chu Chupin. Diabetes mellitus and risk of skeletal fracture[J]. New England Journal of Medicine, 1980, 303(10): 567-570.
[59] Macey L R, Kana S M, Jingushi S, et al. Defects of early fracture-healing in experimental diabetes[J]. The Journal of Bone and Joint Surgery, 1989, 71(5): 722-733.
[60] Hofbauer L C, Brueck C C, Singh S K, et al. Osteoporosis in patients with diabetes mellitus[J]. Journal of Bone & Mineral Research, 2007, 22(9): 1317-1328.
[61] Gulcelik N E, Bayraktar M, Caglar O, et al. Mortality after hip fracture in diabetic patients[J]. Experimental and Clinical Endocrinology & Diabetes, 2011, 119(7): 414-418.
[62] Hanft J R, Landsman A, Surprenant M S, et al. The role of combined magnetic field (CMF) bone growth stimulator as an adjunct in the treatment of neuroarthropathy Charcot joint[J]. Diabetes, 1998, 37(6): 510-515.
[63] Zhang Hao, Gan Lu, Zhu Xiaoquan, et al. Moderate- intensity 4mT static magnetic fields prevent bone architectural deterioration and strength reduction by stimulating bone formation in streptozotocin-treated diabetic rats[J]. Bone, 2018, 107: 36-44.
[64] Jing Da, Cai Jing, Shen Guanghao, et al. The preventive effects of pulsed electromagnetic fields on diabetic bone loss in streptozotocin-treated rats[J]. Osteoporosis International, 2011, 22(6): 1885-1895.
[65] Li Jianjun, Zeng Zhaobin, Zhao Yantao, et al. Effects of low-intensity pulsed electromagnetic fields on bone microarchitecture, mechanical strength and bone turnover in type 2 diabetic db/db mice[J]. Scientific Reports, 2017, 7(1): 1-13.
[66] Cai Jing, Li W, Sun Tingyi, et al. Pulsed electro- magnetic fields preserve bone architecture and mechanical properties and stimulate porous implant osseointegration by promoting bone anabolism in type 1 diabetic rabbits[J]. Osteoporosis International, 2018, 29(5): 1177-1191.
[67] Dean P M, Matthews E K, Sakamoto Y. Pancreatic islet cells: effects of monosaccharides, glycolytic intermediates and metabolic inhibitors on membrane potential and electrical activity[J]. The Journal of Physiology, 1975, 246(2): 459-478.
[68] Lahbib A, Ghodbane S, Louchami K, et al. Effects of vitamin D on insulin secretion and glucose transporter GLUT2 under static magnetic field in rat[J]. Environmental Science & Pollution Research, 2015, 22(22): 18011-18016.
[69] Elferchichi M, Mercier J, Ammari M, et al. Subacute static magnetic field exposure in rat induces a pseudoanemia status with increase in MCT4 and Glut4 proteins in glycolytic muscle[J]. Environmental Science & Pollution Research, 2016, 23(2): 1265- 1273.
[70] Laszlo J F, Szilvasi J, Fenyi A, et al. Daily exposure to inhomogeneous static magnetic field significantly reduces blood glucose level in diabetic mice[J]. International Journal of Radiation Biology, 2011, 87(1): 36-45.
[71] Hirose H, Nakahara T, Zhang Qiumei, et al. Static magnetic field with a strong magnetic field gradient (41.7T/m) induces c-Jun expression in HL-60 cells[J]. Vitro Cellular & Developmental Biology Animal, 2003, 39(8-9): 348-352.
[72] Abdelmelek H, Molnar A, Servais S, et al. Skeletal muscle HSP72 and norepinephrine response to static magnetic field in rat[J]. Journal of Neural Trans- mission, 2006, 113(7): 821-827.
[73] Mcleod B R, Liboff A R, Smith S D. Electromagnetic gating in ion channels[J]. Journal of Theoretical Biology, 1992, 158(1): 15-31.
[74] Tenforde T S. Biological interactions of extremely- low-frequency electric and magnetic-fields[J]. Journal of Electroanalytical Chemistry and Interfacial Elec- trochemistry, 1991, 25(1): 1-17.
[75] Iwasaka M, Ueno S. Magnetic-field parallel motion of living cells[J]. Journal of Applied Physics, 2005, 97(10): 377-380.
[76] Ueno S, Iwasaka M, Kitajima T. Redistribution of dissolved-oxygen concentration under magnetic-fields up to 8-T[J]. Journal of Applied Physics, 1994, 75(10): 7174-7176.
[77] Carter C S, Huang S C, Searby C C, et al. Exposure to static magnetic and electric fields treats type 2 diabetes[J]. Cell Metabolism, 2020, 32(4): 561-574.
Research Progress of the Effects of Magnetic Field on Blood Glucose and Diabetic Complications
Feng Chuanlin1,2 Yu Biao2,3 Fang Yanwen4 Fang Zhicai4 Zhang Xin1,2,3
(1. Institutes of Physical Science and Information Technology Anhui University Hefei 230601 China 2. High Magnetic Field Laboratory Hefei Institutes of Physical Science Chinese Academy of Sciences Hefei 230031 China 3. Science Island Branch of Graduate School University of Science and Technology of China Hefei 230026 China 4. Heye Health Technology Co. Ltd Huzhou 313300 China)
Abstract Several studies have shown that magnetic fields have various effects on the blood glucose level and diabetic complications. This paper systematically compares and analyzes the effects of magnetic fields with different parameters on blood glucose, insulin levels, as well as diabetes complications in different biological systems. The results show that although the effects of magnetic fields on blood glucose and insulin levels are variable due to the diversity of magnetic parameters and biological samples examined, the magnetic fields usually have positive effects on diabetic wound healing and osteoarthropathy, indicating their promising clinical application potential. Although the mechanism of magnetic field affecting blood glucose has not been systematically explored, there are evidences showing that magnetic field may affect insulin levels by affecting cell membrane, membrane proteins and Ca2+ concentrations. This will not only help people to understand the effect of magnetic field on blood glucose regulation and diabetes, but also lay a foundation for the systematic investigations and potential application of magnetic field in the treatment of diabetes and its complications in the future.
keywords:Magnetic field, blood glucose, insulin, diabetes mellitus, diabetic complications
中图分类号:Q689
DOI: 10.19595/j.cnki.1000-6753.tces.201257
中科院合肥物质科学研究院院长基金资助项目(YZJJ201704)。
收稿日期 2020-09-20
改稿日期 2020-09-27
作者简介
冯传林 男,1997年生,硕士研究生,研究方向为稳态磁场对糖尿病小鼠的影响及机制。E-mail: fengcl@yeah.net
张 欣 女,1979年生,研究员,博士生导师,研究方向为不同参数稳态和低频磁场的生物学效应、机制及其生物医学应用。E-mail: xinzhang@hmfl.ac.cn (通信作者)
(编辑 崔文静)
 发病;②Ⅱ型糖尿病(Diabetes Mellitus Type 2, T2DM),特点是胰岛素合成和分泌不足,约占全世界所有糖尿病的90%,其发病率可随着年龄的增长而增加;③妊娠糖尿病(Gestational Diabetes Mellitus, GDM),指的是妊娠期间发生并在妊娠期结束时消退的糖尿病;④青年成熟期发病型糖尿病(Maturity Onset Diabetes of the Young, MODY),是一种单基因型糖尿病,只占糖尿病患者总数很小的一部分,一般在20岁左右被诊断,与基因突变有 关[9]。此外,一些疾病和化学药物,如胰腺炎、甲状腺机能亢进、蛋白酶抑制剂和噻嗪类利尿剂也会造成一些继发性糖尿病的产生[9]。
发病;②Ⅱ型糖尿病(Diabetes Mellitus Type 2, T2DM),特点是胰岛素合成和分泌不足,约占全世界所有糖尿病的90%,其发病率可随着年龄的增长而增加;③妊娠糖尿病(Gestational Diabetes Mellitus, GDM),指的是妊娠期间发生并在妊娠期结束时消退的糖尿病;④青年成熟期发病型糖尿病(Maturity Onset Diabetes of the Young, MODY),是一种单基因型糖尿病,只占糖尿病患者总数很小的一部分,一般在20岁左右被诊断,与基因突变有 关[9]。此外,一些疾病和化学药物,如胰腺炎、甲状腺机能亢进、蛋白酶抑制剂和噻嗪类利尿剂也会造成一些继发性糖尿病的产生[9]。