0 引言
SF6绝缘气体因其良好的绝缘和灭弧性能,已被广泛应用于多种高压电气设备中[1-4]。但SF6气体绝缘设备在安装、运行、调试和检修过程中,不可避免地会有SF6气体泄漏到大气中。然而,SF6是一种温室效应极强的气体,其温室效应是CO2的23 900倍,是CH4的1 140倍[5-6],并且在大气中极难降解。随着《巴黎协定》的签署,为了实现在21世纪后半叶温室气体的净零排放目标,采用电气性能和优良的气体替代SF6,已成为电力工业亟待解决的问题。
从20世纪70年代开始,各国学者就开始了新型SF6环保型代气体的研究,并发现C3F7CN、C5F10O、C6F12O和CF3I等环保气体具有良好的绝缘性能,在一定程度上可替代SF6[7-13]。其中,电负性气体CF3I因具有稳定、绝缘能力强、热传导和灭弧水平接近SF6等突出优势,受到了广泛的关注[14-15]。但CF3I会在局部放电(Partial Discharge,PD)情况下发生分解,并进一步与气室中不可避免存在的微量H2O和微量O2反应,生成C2F6、I2、C2F5I、HF、C2F4和CH3I等[16-18]。其中,C2F6的含量最多,CH3I则在放电达到一定程度时才会产生[16]。一方面这些分解产物含量过多会降低气体绝缘设备的绝缘水平;另一方面它们也能有效反映PD程度和故障情况。因此,有必要对典型PD产物含量进行监测。
由于基于单壁碳纳米管(Single Walled Carbon Nanotube, SWCNT)的气敏传感器具有响应好、体积小和灵敏度高等突出优点,近年来被广泛关注。此外,通过在其表面修饰过渡金属,SWCNT的气敏特性将在原有基础上显著提高。其中,掺杂对分子具有较高催化活性的Pt可实现对某些不常见气体的检测[19-24]。Cui Hao等学者通过仿真发现,由于吸附材料与目标气体之间存在较强的轨道相互作用和化学吸附,在表面修饰有Pt、Pd和Rh的SWCNT对SOF2、CO、CH4和H2S具有良好的气敏特性[25-27]。M.Yoosefian[28]则研究了SO2在Pt和Au掺杂的(5, 5)SWCNT上的吸附特性,并发现SO2在Pt-SWCNT上的吸附能隙比在Au-SWCNT上的变化更大。
为此,本文基于密度泛函理论(Density Functional Theory,DFT),探究了C2F6和CH3I两种典型CF3I的PD分解产物在Pt修饰(8, 0) SWCNT上的吸附参数、态密度和前沿分子轨道等吸附特性,为环保型气体CF3I在气体绝缘设备中的最终运用和监测提供理论依据。
1 计算参数
本研究采用Dmol3量子化学模块进行DFT计算[29]。采用广义密度近似方法(Generalized Gradient Approximation, GGA)的Perdew-Burke-Ernzerho(PBE)函数处理电子交换关联作用[30]。采用双数值p极化(Double Numerical plus Polarization, DNP)作为原子轨道基组。最大原子位移、能量收敛精度、能量梯度和轨道拖尾效应分别设置为5×10-3Å(1Å=10-10m)、1.0×10-5Ha(1Ha=27.211eV)、0.002Ha/Å和0.005Ha[31]。此外,为保证总能量的计算精度,自洽场误差和全球轨道截止半径选为1.0×10−6Ha和5.0Å[32]。布里渊k点网格空间设置为1×1×8[33]。使用DFT-D(Tkatchenk and Scheffler, TS)方法处理色散力[34]。吸附过程中的电荷转移量 TQ通过Mulliken法获得[35]。若电荷转移量 TQ>0,表示电子从气体分子转移到Pt-SWCNT表面;反之,若电荷转移量TQ<0,则表示电子从Pt-SWCNT表面转移到气体分子。此外,定义吸附能Eg为
式中,Egas为单个气体分子的所具有能量;EPt-SWCNT为未吸附气体分子时的本征Pt-SWCNT的能量;Egas/Pt-SWCNT为气体分子吸附在Pt-SWCNT表面后的总的能量。若Eg>0,则代表该吸附过程中,整个体系从外界吸附能量;反之,若Eg<0,则代表该吸附过程中,整个体系向外界释放能量。
2 结果分析
在进行吸附计算前,本研究首先优化了CH3I、C2F6和Pt-SWCNT的结构。其中,选择具有20Å×20Å×8.5Å的超胞作为Pt掺杂(8,0)SWCNT的纳米载体。Pt原子则与SWCNT上两个相邻的C原子同时相连,在SWCNT外形成桥位[35]。Pt掺杂后并未改变SWCNT的结构,C-C键键长仍保持为1.439Å,而Pt-C键键长为2.265Å,如图1所示。Pt掺杂前后的总态密度(Total Density of State,TDOS)如图2所示。Pt掺杂后,在费米能级左面的-1.25~-0.5eV范围内出现了一个波峰,这主要由掺杂的Pt原子所贡献。同时,在掺杂过程中,Pt向SWCNT转移了0.200e电子。优化后的C2F6分子和CH3I分子如图3所示。C2F6分子中C-C键和C-F键的键长分别为1.560Å和1.348Å;F-C-F和F-C-C的键角分别为109.037°和109.894°。对于CH3I分子,C-H键和C-I键的键长分别为1.092Å和2.187Å;H-C-H和H-C-I的键角则分别为111.664°和107.185°。

图1 Pt掺杂SWCNT优化结构
Fig.1 The optimized structure of Pt-doped SWCNT

图2 Pt掺杂SWCNT前后的总态密度
Fig.2 The TDOS of Pt-SWCNT and intrinsic SWCNT
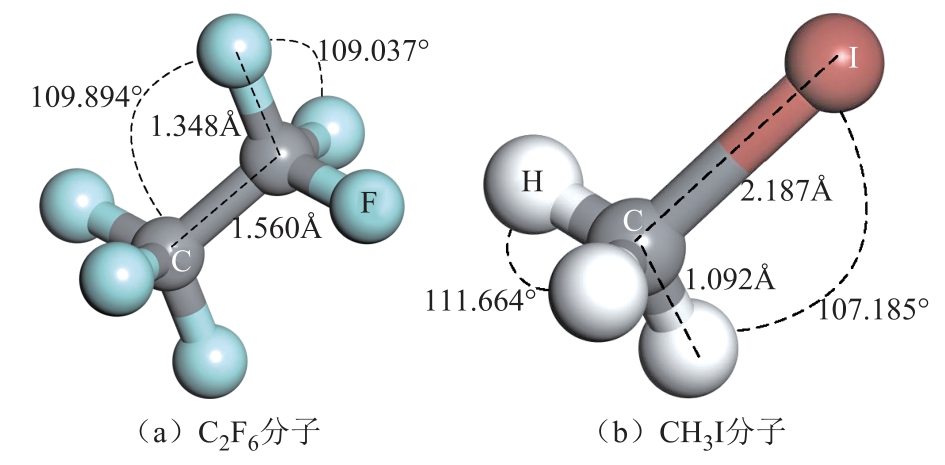
图3 气体分子优化结构
Fig.3 The optimized structure of gas molecules
2.1 C2F6分子和CH3I分子在Pt-SWCNT表面的吸附特征参数
C2F6分子和CH3I分子分别吸附在Pt-SWCNT表面的最优化结构如图4和图5所示,吸附能等吸附参数见表1。由于C2F6分子呈轴对称及两端对称性,仅考虑一种初始吸附结构,即C2F6分子的一个F原子靠近Pt原子。由图4优化后的C2F6分子吸附于Pt-SWCNT表面的最优结构可知,该体系的吸附能为0.223eV,即在吸附过程中整个体系会吸收0.223eV的能量。吸附距离为2.769Å,且在吸附过程中有0.016e的电子从C2F6分子转移到Pt-SWCNT表面。此外,Pt-SWCNT和C2F6分子的键长键角在吸附后也未发生变化。考虑到较小的吸附能和电荷转移量,可以认为Pt-SWCNT和C2F6分子之间的相互作用较弱。
表1 C2F6分子和CH3I分子在Pt-SWCNT表面上的吸附参数
Tab.1 Adsorption parameters of C2F6 and CH3I molecules on the surface of Pt-SWCNT

?

图4 C2F6吸附在Pt-SWCNT表面的最优结构示意图Fig.4 The optimized configuration of C2F6 adsorbed on the surface of Pt-SWCNT

图5 CH3I吸附在Pt-SWCNT表面的最优结构示意图
Fig.5 The optimized configuration of CH3I adsorbed on the surface of Pt-SWCNT
对于呈轴对称的CH3I分子,本文考虑了两种初始吸附结构:一种为CH3I分子的一个H原子靠近Pt原子(简写为CH3I-H);另一种为CH3I分子的I原子靠近Pt原子(简写为CH3I-I)。由图5优化后的C2F6分子吸附于Pt-SWCNT表面的最优结构可知,对于CH3I-H吸附结构,该体系在吸附过程中吸收了0.132eV能量,吸附距离为2.275Å,同时在吸附过程中有0.070e的电子从CH3I分子转移到Pt-SWCNT表面上。吸附后的Pt-SWCNT和CH3I分子的结构(键长和键角)未发生改变。对于CH3I-I吸附结构,其中一个Pt-C键断裂并与CH3I分子的I原子形成键长为2.728Å的Pt-I键。但相较于吸附前的CH3I分子,吸附后的CH3I分子结构并未发生显著变化。与此同时,该体系的吸附能远远大于CH3I-H吸附结构的吸附能,达到了0.772eV,并有0.192e的电子在Pt-I键的成键过程中从CH3I分子转移到Pt-SWCNT表面。
2.2 态密度分析
C2F6分子吸附体系的TDOS和分波态密度(Partial Density of State, PDOS)如图6所示。其中,“Pt-SWCNT”代表未吸附气体分子的本征Pt-SWCNT的TDOS,“C2F6吸附”则代表C2F6在Pt-SWCNT表面达到稳定状态时整个体系的TDOS。由图6可知,相较于本征Pt-SWCNT,吸附C2F6后的TDOS在-16eV、-13 eV、-11.5 eV、-10.5 eV和-6eV处显著增大,但在费米能级处的TDOS变化很小。此外,根据PDOS可知,Pt-SWCNT吸附C2F6分子后,Pt原子的5d轨道和C2F6分子中C和F原子的2p轨道的重合区较小,这进一步表明Pt-SWCNT和C2F6分子间的相互作用可能较弱。
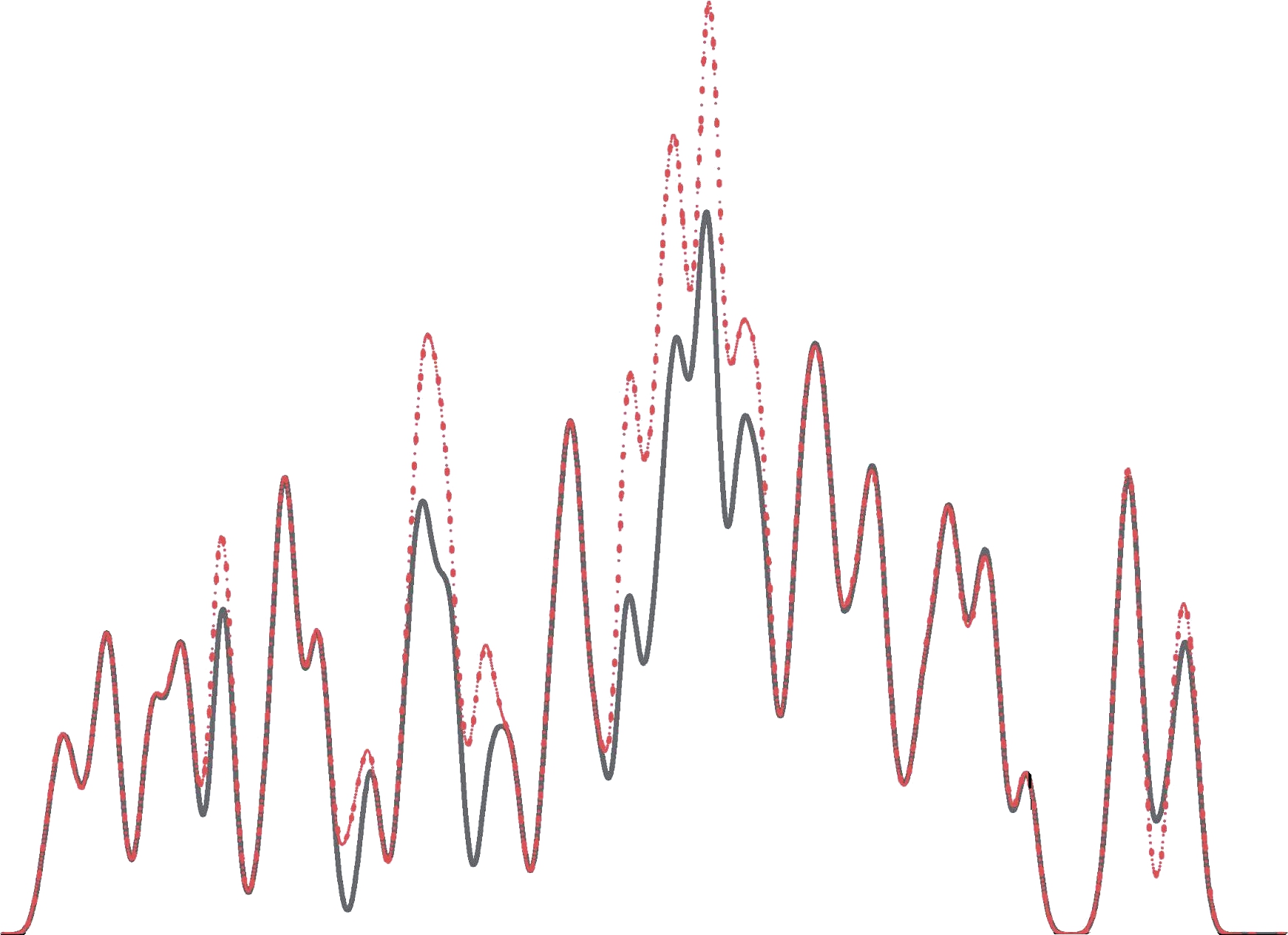

图6 C2F6在Pt-SWCNT表面的稳定体系的态密度
Fig.6 The density of states of C2F6 absorbed on the surface of Pt-SWCNT
CH3I分子吸附体系的TDOS和PDOS如图7和图8所示。由图7可知,对于CH3I-H吸附结构,吸附CH3I分子后,整个体系的TDOS与本征Pt-SWCNT的差异并不显著;吸附CH3I分子后的PDOS,Pt原子的5d轨道仅与CH3I分子中I原子的5p轨道在-1.25eV处有一定的重叠,吸附前后体系的导电性变化不大。由图8可知,相较于本征Pt-SWCNT的TDOS,CH3I-I的稳定体系的TDOS发生显著变化,带隙和费米能级处的TDOS也明显减小。在-3.75~-2.0eV处,CH3I中I原子的5p轨道和Pt-SWCNT中Pt原子的5d轨道面积有一定的重叠。这表示Pt-SWCNT材料按CH3I-I吸附结构吸附CH3I分子后的导电性有所增强。

图7 CH3I-H的稳定体系的态密度
Fig.7 The density of states of CH3I-H
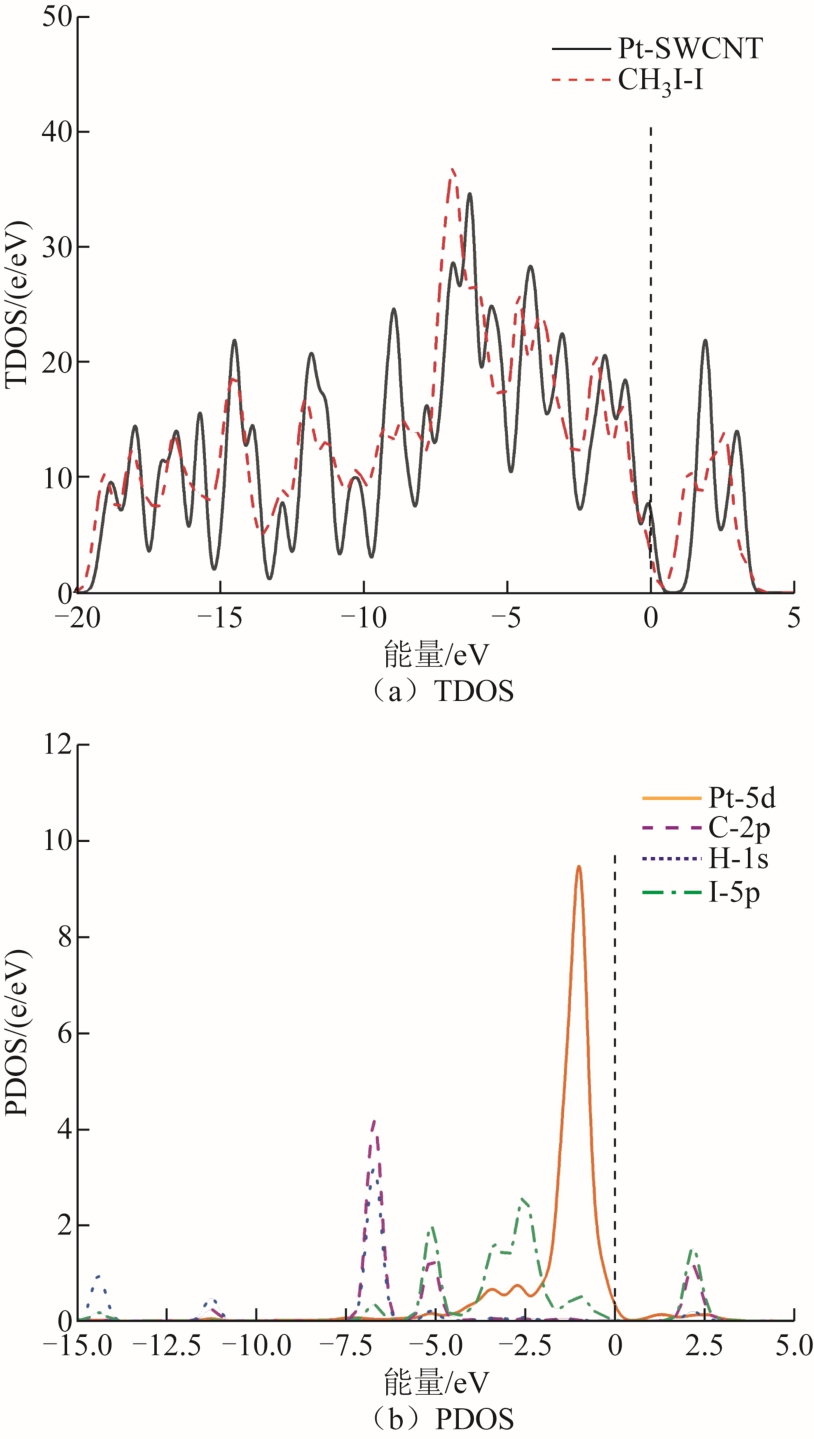
图8 CH3I-I的稳定体系的态密度
Fig.8 The density of states of CH3I-I
2.3 前沿分子轨道理论分析
基于前沿分子轨道理论,本文得到了本征Pt-SWCNT表面、C2F6分子吸附体系及CH3I分子吸附体系的最高占据轨道(Highest Occupied Molecular Orbital, HOMO)和最低未占据轨道(Lowest Unoccupied Molecular Orbital,LUMO)的能量数值及分布,如图9~图11所示。其中能隙Ea=LUMOHOMO。在本征Pt-SWCNT中,LUMO和HOMO分别为-4.377eV和-5.083eV,能隙为0.706eV。在C2F6吸附于Pt-SWCNT表面后,该体系的LUMO和HOMO分别变为-4.335eV和-5.048eV,均略大于本征Pt-SWCNT的;而该体系能隙仅略微增大至0.713eV;此外,LUMO和HOMO未发生明显的变化。在CH3I分子吸附于Pt-SWCNT表面后的LUMO和HOMO则如图11所示。对于CH3I-H吸附结构,吸附CH3I分子后体系的LUMO、HOMO和能隙分别为-4.434eV、-5.151eV和0.717eV。LUMO和HOMO均略小于本征Pt-SWCNT的,但能隙略微大于本征Pt-SWCNT的;此外,LUMO和HOMO的分布在吸附前后也未发生明显的变化。对于CH3I-I吸附结构,吸附CH3I分子后体系的LUMO、HOMO和能隙分别为-4.557eV、-5.002eV和0.445eV,能隙在吸附后减小了0.261eV,并且HOMO基本分布于Pt原子和CH3I分子上。若能隙变大,表示体系的导电性变弱,而能隙变小则体系的导电性会变强[36]。因此,在宏观上,按CH3I-I吸附结构吸附CH3I分子后的Pt-SWCNT的导电性可能会显著增大;吸附C2F6分子后的Pt-SWCNT和按CH3I-H吸附结构吸附CH3I分子后的Pt-SWCNT的导电性均可能会发生略微的减小。

图9 Pt-SWCNT的HOMO和LUMO分布及能量
Fig.9 HOMO and LUMO distribution and relative energies for Pt-SWCNT
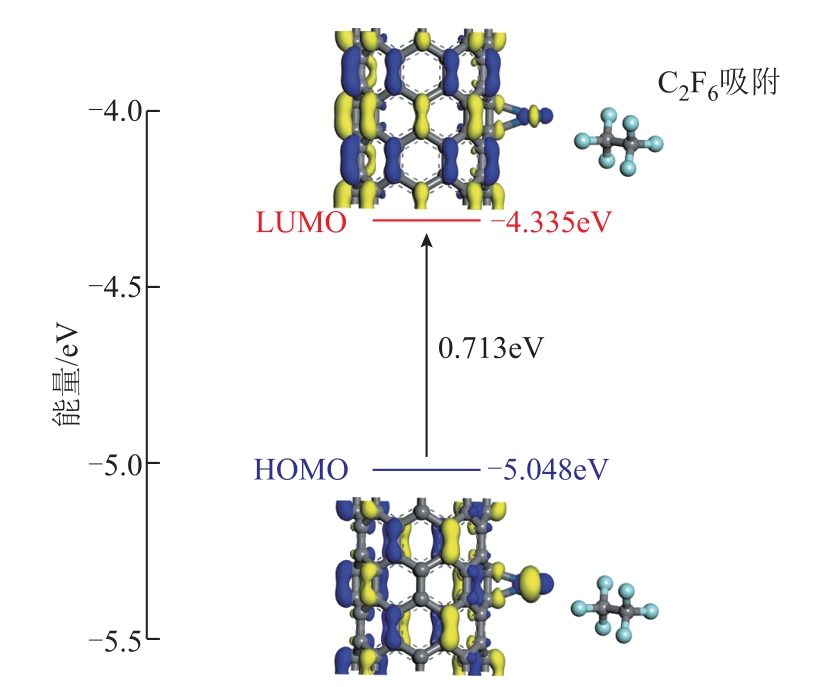
图10 C2F6吸附体系的HOMO和LUMO分布及能量
Fig.10 HOMO and LUMO distribution and relative energies of C2F6 adsorbed on Pt-SWCNT

图11 CH3I吸附体系的HOMO和LUMO分布及能量
Fig.11 HOMO and LUMO distribution and relative energies of CH3I adsorbed on Pt-SWCNT
3 结论
本文基于DFT,研究了C2F6和CH3I两种典型CF3I PD分解产物在Pt-SWCNT上的吸附特征参数具体结论如下:
1)Pt-SWCNT和C2F6分子间的吸附能及电荷转移量均很小,并且Pt-SWCNT在C2F6分子吸附前后的态密度、LUMO、HOMO及能隙变化并不明显,表明Pt-SWCNT和C2F6分子之间相互作用很弱,Pt-SWCNT并不适用于检测C2F6气体。
2)对于CH3I,本文考虑了两种初始吸附结构,综合分析吸附参数、态密度和前沿分子轨道,Pt-SWCNT更可能按CH3I-I吸附结构吸附CH3I分子。此外,Pt-SWCNT和CH3I分子之间有着较强的相互作用,并且该吸附以化学吸附为主。由于吸附CH3I后能隙减小了0.261eV,使得Pt-SWCNT的导电性的增加,可认为Pt-SWCNT对CH3I有着较好的气敏特性。
简而言之,Pt-SWCNT可用于检测CH3I气体,但对C2F6的气敏特性较差。这为环保型气体CF3I在气体绝缘设备中的监测提供理论依据。
[1] 李亚龙, 张晓星, 崔兆仑, 等. NH3对DBD降解SF6影响的试验研究[J]. 电工技术学报, 2019, 34(24):5262-5269.Li Yalong, Zhang Xiaoxing, Cui Zhaolun, et al.Experiment of effect of ammonia on degradation of sulfur hexafluoride by dielectric barrier discharge[J].Transactions of China Electrotechnical Society, 2019,34(24): 5262-5269.
[2] 周朕蕊, 韩冬, 赵明月, 等. SF6替代气体分解特性的研究综述[J]. 电工技术学报, 2020, 35(23): 4998-5014.Zhou Zhenrui, Han Dong, Zhao Mingyue, et al.Review on decomposition characteristics of SF6 alternative gases[J]. Transactions of China Electrotechnical Society, 2020, 35(23): 4998-5014.
[3] Wei Gang, Bai Yichun, Hu Ming, et al. Research on environmental protection treatment for ex-service SF6 adsorbent[J]. IEEE Access, 2020, 8: 93840-93849.
[4] Rabie M, Franck C M. Assessment of eco-friendly gases for electrical insulation to replace the most potent industrial greenhouse gas SF6[J].Environmental Science & Technology, 2018, 52(2):369-380.
[5] 侯志强, 郭若琛, 李军浩. 直流电压下SF6/N2混合气体沿面局部放电特性[J]. 电工技术学报, 2020,35(14): 3087-3096.Hou Zhiqiang, Guo Ruochen, Li Junhao. Partial discharge characteristics of the surface discharge in SF6/N2 of the mixed gas under DC voltage[J].Transactions of China Electrotechnical Society, 2020,35(14): 3087-3096.
[6] Smeets RPP, Kertesz V, Dufournet D, et al. Interaction of a vacuum arc with an SF6 arc in a hybrid circuit breaker during high-current interruption[J]. IEEE Transactions on Plasma Science, 2007, 35(4): 933-938.
[7] Xiao Meng, Feng Xiaoman, Li Xiayu, et al. Effect of micro-H2O and micro-O2 on the decomposition characteristics of insulating medium C3F7CN gas using molecular dynamics and transition state method[J].Journal of Molecular Modeling, 2020, 26: 252.
[8] 唐炬, 雷志城, 万兆丰, 等. 环保型C6F12O气体绝缘介质过热分解的反应热力学特性[J]. 中国电机工程学报, 2019, 39(17): 5257-5262.Tang Ju, Lei Zhicheng, Wan Zhaofeng, et al. Reaction thermodynamics of overthermal decomposition of C6F12O[J]. Proceeding of the CESS, 2019, 39(17):5257-5262.
[9] Kieffel Y, Irwin T, Ponchon P, et al. Green gas to replace SF6 in electrical grids[J]. IEEE Power Energy Magazine SI, 2016, 14(2): 32-39.
[10] Zhang Yue, Zhang Xiaoxing, Li Yi, et al. AC breakdown and decomposition characteristics of environmental friendly gas C5F10O/air and C5F10O/N2[J].IEEE Access, 2019, 7: 73954-73960.
[11] 周朕蕊, 韩冬, 赵明月, 等. 电晕放电下C5F10O混合气体的分解特性[J]. 电工技术学报, 2021, 36(2):407-416.Zhou Zhenrui, Han Dong, Zhao Mingyue, et al.Decomposition characteristics of C5F10O mixture under corona discharge[J]. Transactions of China Electrotechnical Society, 2021, 36(2): 407-416.
[12] Deng Yunkun, Xiao Dengmin. Analysis of the insulation characteristics of CF3I gas mixtures with Ar,Xe, He, N2 and CO2 using Boltzmann equation method[J]. Japanese Journal of Applied Physics, 2014,53(9): 3253-3259.
[13] 颜湘莲, 郑宇, 黄河, 等. C4F7N/CO2混合气体对局部不均匀电场的敏感特性[J]. 电工技术学报, 2020,35(1): 43-51.Yan Xianglian, Zheng Yu, Huang He, et al. Sensitivity of C4F7N/CO2 gas mixture to partial inhomogeneous electric field[J]. Transactions of China Electrotechnical Society, 2020, 35(1): 43-51.
[14] Katagiri H, Kasuya H, Mizoguchi H, et al.Investigation of the performance of CF3I gas as a possible substitute for SF6[J]. IEEE Transactions on Dielectrics and Electrical Insulation, 2008, 15(5):1424-1429.
[15] Cressault Y, Connord V, Hingana H, et al. Transport properties of CF3I thermal plasmas mixed with CO2,air or N2 as an alternative to SF6 plasmas in high voltage circuit breakers[J]. Journal of Physics D:Applied Physics, 2011, 44(49): 1-9.
[16] 张晓星, 田双双, 肖淞, 等. SF6替代气体研究现状综述[J]. 电工技术学报, 2018, 33(12): 2883-2893.Zhang Xiaoxing, Tian Shuangshuang, Xiao Song, et al.A review study of SF6 substitute gases[J].Transactions of China Electrotechnical Society, 2019,39(17): 5257-5262.
[17] 张晓星, 戴琦伟, 韩晔飞, 等. CF3I在微水条件下的放电分解组分研究[J]. 高电压技术, 2016, 42(1):172-178.Zhang Xiaoxing, Dai Qiwei, Han Yefei, et al.Investigation towards the influence of trace water on CF3I decomposition components under discharge[J].High Voltage Engineering, 2016, 42(1): 172-178.
[18] Zhang Xiaoxing, Xiao Song, Zhang Jian, et al.Influence of humidity on the decomposition products and insulating characteristics of CF3I[J]. IEEE Transactions on Dielectrics & Electrical Insulation,2016, 23(2): 819-828.
[19] Yoosefian M, Etminan N. The role of solvent polarity in the electronic properties, stability and reactivity trend of a tryptophane/Pd doped SWCNT novel nanobiosensor from polar protic to non-polar solvents[J]. RSC Advances, 2016, 69(6): 64818-64825.
[20] Nazanin Etminan, Mehdi Yoosefian, Heidar Raissi, et al. Solvent effects on the stability and the electronic properties of histidine/Pd-doped single-walled carbon nanotube biosensor[J]. Journal of Molecular Liquids,2016, 214: 313-318.
[21] Robinson J A, Snow E S, Ba C, et al. Role of defects in single-walled carbon nanotube chemical sensors[J].Nano Letters, 2006, 6(8): 1747-1751.
[22] Yoosefian M. A high efficient nanostructured filter based on functionalized carbon nanotube to reduce the tobacco-specific nitrosamines[J]. Applied Surface Science, 2018, 434: 134-141.
[23] Peng X, Meguid S A. Molecular dynamics simulations of the buckling behavior of defective carbon nanotubes embedded in epoxy nanocomposites[J].European Polymer Journal, 2017, 93: 246-258.
[24] Zhang Xiaoxing, Cui Hao, Chen Dachang, et al.Electronic structure and H2S adsorption property of Pt3 cluster decorated(8, 0) SWCNT[J]. Applied Surface Science, 2018, 428: 82-88.
[25] Cui Hao, Zhang Xiaoxing, Chen Dachang, et al. Pt &Pd decorated CNT as a workable media for SOF2 sensing: a DFT study[J]. Applied Surface Science,2019, 471: 335-341.
[26] Larciprete R, Petaccia L, Lizzit S, et al. The role of metal contact in the sensitivity of single-walled carbon nanotubes to NO2[J]. The Journal of Physical Chemistry C, 2007, 111(33): 12169-12174.
[27] Cui Hao, Zhang Xiaoxing, Zhang Jun, et al.Interaction of CO and CH4 adsorption with noble metal (Rh, Pd, and Pt)-decorated N3‑CNTs: a firstprinciples study[J]. ACS Omega, 2018, 3: 16892-16898.
[28] Yoosefian M, Zahedi M, Mola A, et al. DFT comparative study of single and double SO2 adsorption on Pt-doped and Au-doped single-walled carbon nanotube[J]. Applied Surface Science, 2015,349: 864-869.
[29] Delley B. From molecules to solids with the DMol3 approach[J]. The Journal of Chemical Physics, 2000,113(18): 7756-7764.
[30] Perdew J P, Burke K, Ernzerhof M. Generalized gradient approximation made simple[J]. Physical Review Letters, 1996, 77(18): 3865-3868.
[31] Zhang Xiaoxing, Yu Lei, Tie Jing, et al. Gas sensitivity and sensing mechanism studies on Audoped TiO2 nanotube arrays for detecting SF6 decomposed components[J]. Sensors, 2014, 14(10):19517-19532.
[32] Ju Weiwei, Li Tongwei, Su Xiangying, et al. Au cluster adsorption on perfect and defective MoS2 monolayers: structural and electronic properties[J].Physical Chemistry Chemical Physics, 2017, 19(31):20735-20748.
[33] Zhang Xiaoxing, Gui Yingang, Xiao Hanyan, et al.Analysis of adsorption properties of typical partial discharge gases on Ni-SWCNTs using density functional theory[J]. Applied Surface Science, 2016,379: 47-54.
[34] Tkatchenko A, DiStasio R A, Head-Gordon M, et al.Dispersion-corrected Møller–Plesset second-order perturbation theory[J]. The Journal of Chemical Physics, 2009, 131(9): 094106.
[35] Xiao Song, Zhang Jun, Zhang Xiaoxing, et al. Ptdoped single-walled CNT as a superior media for evaluating the operation status of insulation devices: a first-principle study[J]. AIP Advances, 2018, 8(10):105101.
[36] Cui Hao, Zhang Xiaoxing, Zhang Jun, et al.Adsorption behaviour of SF6 decomposed species onto Pd4-decorated single-walled CNT: a DFT study[J].Molecular Physics, 2018, 116(13): 1749- 1755.